
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
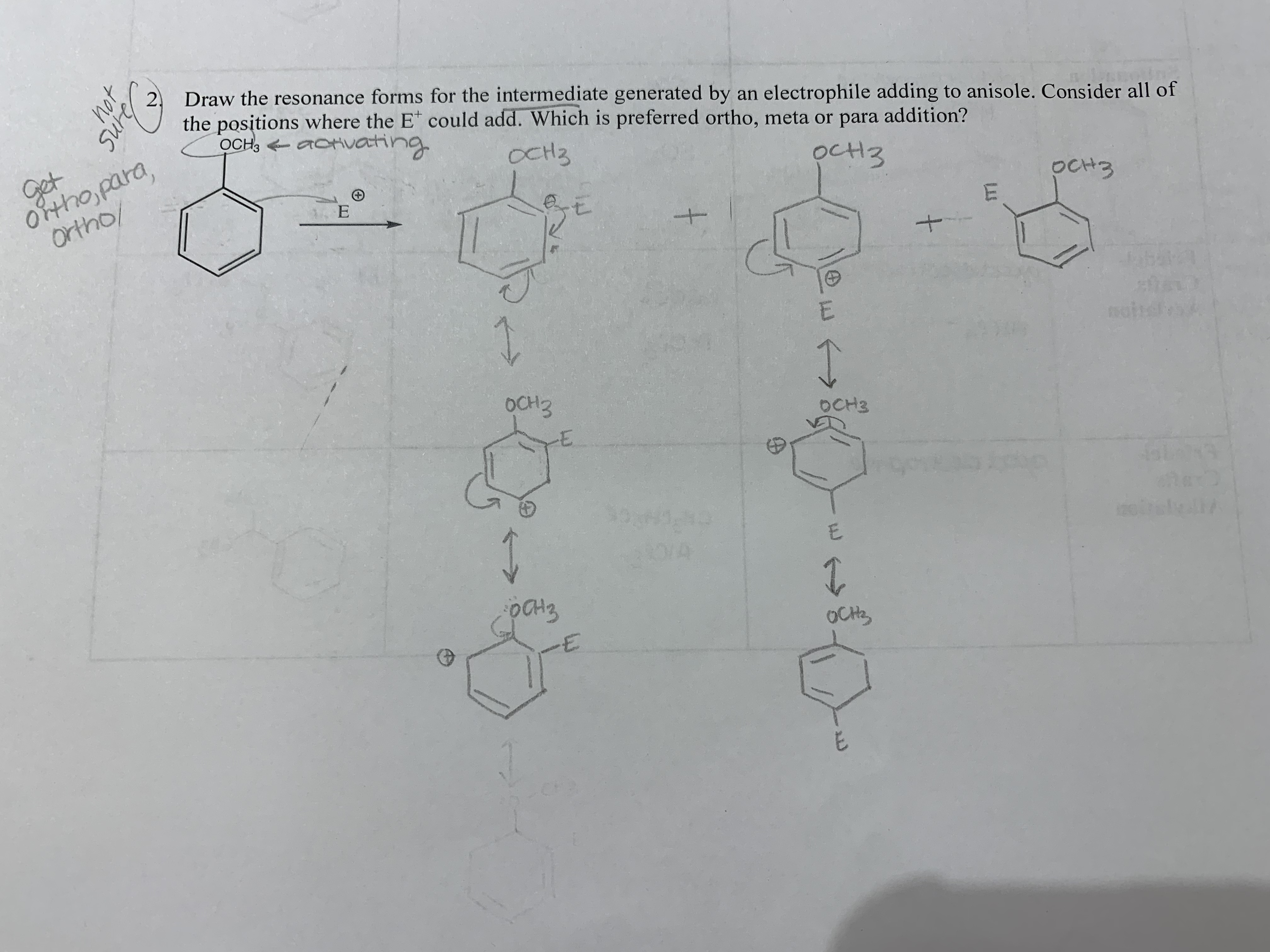
Draw the resonance forms for the intermediate generated by an electrophile adding to anisole. Consider all of the positions where the E+ could add. Which is preferred ortho, meta or para addition?
Is my work correct?
Transcribed Image Text:hot
Sure
రఇ 3
(T
+
2
Draw the resonance forms for the intermediate generated by an electrophile adding to anisole. Consider all of
the positions where the E could add. Which is preferred ortho, meta or para addition?
OCHs aoHvachng
Oftho para,
Ortho/
Get
OCH3
OcH3
E
OCH3
E
1
E
OCH3
E
OCH3
E
E
oCHt
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- This question has multiple parts. Work all the parts to get the most points. Consider the following reaction. Propose a mechanism for this reaction. a Step 1 of 3: Add a proton. MeOH Draw the major organic product. Only show specific types of hydrogen atoms: H₂SO4 • Hydrogen atoms not attached to carbon atoms. • Hydrogen atoms involved in the mechanism. 63 Apply formal charges where appropriate. Assign lone pairs and radical electrons where appropriate. == starting points == X OMe Draw the appropriate electron-flow arrows. Omit + signs between structures. If needed, use the "starting points" menu to revert to the original molecule(s) shown. H 1+ H-O ChemDoodle bStep 2 of 3: Make a bond between a nucleophile and an electrophile.arrow_forwardcan you please do the second one as well?arrow_forwardThe substitution reaction studied here with Chlorobutane and KOH is known to have a second order rate equation meaning the transition state in the slow step involves both nucleophile and electrophile. Knowing that, which of the following statements are true? Select all that apply. A) Adding more Chlorobutane (the electrophile) will not change the rate of the reaction. B Adding more KOH (the nucleophile) will make the reaction go faster. Adding more Chlorobutane (the electrophile) will make the reaction go faster. D) Adding more KOH (the nucleophile) will not change the rate of the reaction. E Adding more KOH (the nucleophile) will make the reaction go slower.arrow_forward
- Draw the next most important resonance structure for the enolate shown. Use curved arrows to show the delocalization of electron pairs in both structures. Include lone pairs of electrons, formal charges, and be sure to draw all hydrogen atoms. You can add condensed hydrogens using the More menu, selecting +H and clicking on the carbon as many times as needed. Draw curved arrows to form the next most important Draw the next most important resonance structure, then resonance structure. draw curved arrows to reform the first structure. Select Draw Rings More Erase Select Draw Rings More Erase C :0 : H,C CH2arrow_forwardDrawing Reaction Mechanism Arrowsarrow_forwardLabel each one as either ortho, meta, or para.arrow_forward
- For each reaction below, decide if the first stable organic product that forms in solution will create a new C - C bond, and check the appropriate box. Next, for each reaction to which you answered "Yes" to in the table, draw this product in the drawing area below. Note for advanced students: for this problem, don't worry if you think this product will continue to react under the current conditions - just focus on the first stable product you expect to form in solution. NH2 Домове Will the first product that forms in this reaction create a new C - C bond? ? O Yes Ο No Will the first product that forms in this reaction create a new C - C bond? Yes ○ No Click and drag to start drawing a structure. : Хarrow_forwardRank the following molecules from #1 (fastest) to #3 slowest in their reaction with Cl2/FeCl3.arrow_forwardOrganic Chemistry presented by Macmillan Learning Maxwell Complete the electron-pushing mechanism for the given reaction of 3-pentanone in potassium cyanide and hydrogen cyanide. Add any missing atoms, bonds, charges, nonbonding electron pairs, and curved arrows. Details count. Step 1: add curved arrows. Select Draw Templates More C H H O|N|K] N N: Erase Step 2: complete the structure and add curved arrows. Select Draw Templates More : 0: C H O N H K N: Erasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY