
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
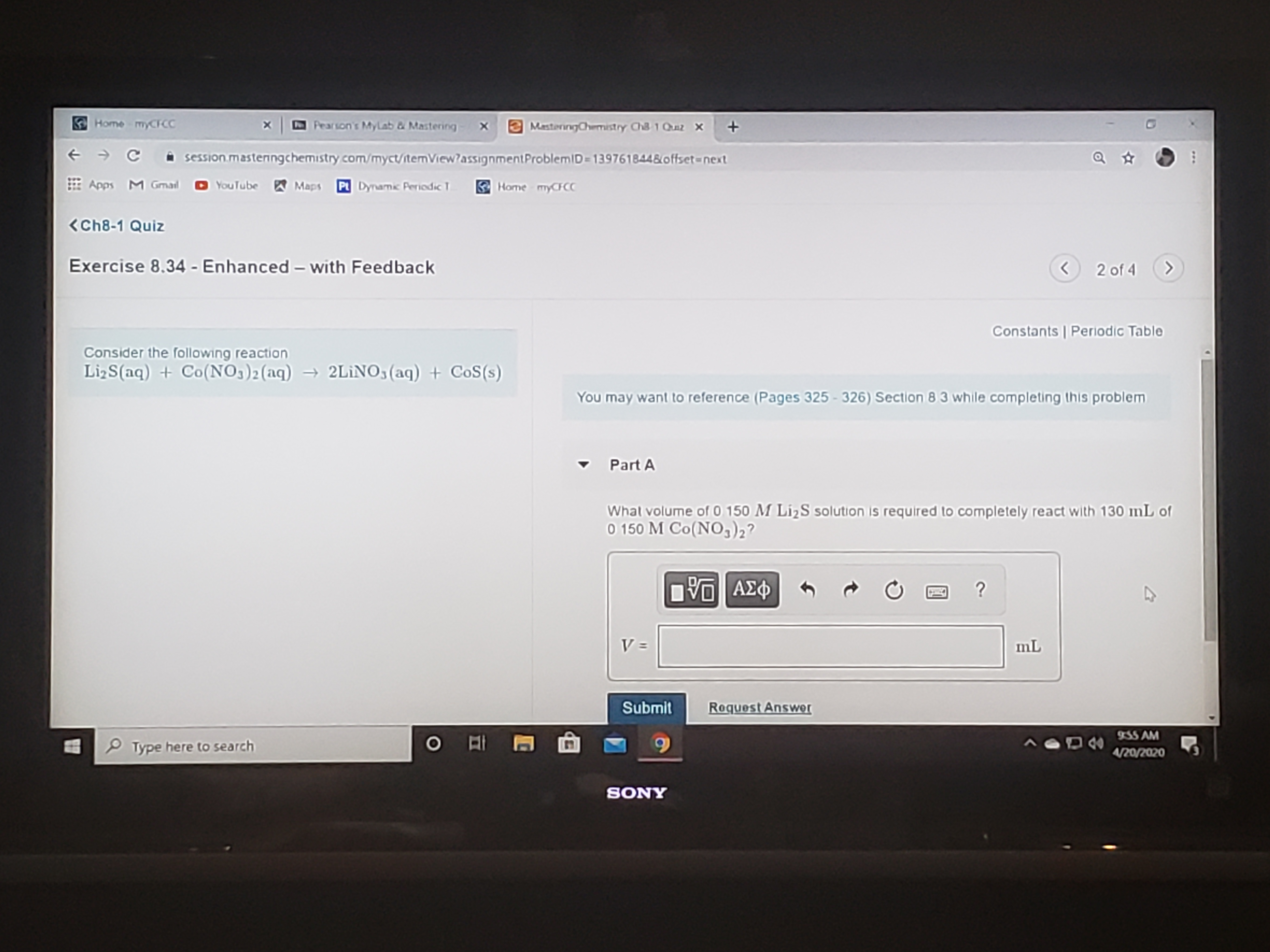
What volume of 0.150 M Li2S solution is required to completely react with 130ml of 0.150 M Co(NO3)2?
Transcribed Image Text:Home mCFCC
Pearson's MyLab & Mastering
e MasterngChmistry Ch 1 Quz x
->
session.masteringchemistry com/myct/ſtemView?assignmentProblemID=139761844&offset=next
E Apps M Gmal
YouTube Maps P Dynamic Periodic T
Home myCCC
<Ch8-1 Quiz
Exercise 8.34 - Enhanced – with Feedback
2 of 4
Constants | Periodic Table
Consider the following reaction
LizS(aq) + Co(NO3)2(aq) → 2LINO3(aq) + CoS(s)
You may want to reference (Pages 325 - 326) Section 8 3 while completing this problem
Part A
Whal volume of 0 150 M LizS solution is required to completely react with 130 mL of
O 150 M Co(NO,)2?
mL
Submit
Roquest Answer
935 AM
P Type here to search
4/20/2020
SONY
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student determined the concentration of NaOCI in a commercial bleach sample using the same technique described in section "F" of the Halogens and Their Compounds lab. The student required 18 drops of 0.01M Na,S203 to reach the end point. What is the concentration (reported in molarity) of NaOCI in the bleach sample?arrow_forwardIt takes 23.98mLs of 0.05M HCl to react completely with 30.00mLs of a barium hydroxide solution. What is the molarity of the barium hydroxide solution?arrow_forward3) Determine the mass of solute in 3.60 L of 0.200 M acetic acid, HC,H3O2.arrow_forward
- How many grams of Ni(OH)2Ni(OH)2 are produced from the reaction of 27.0 mLmL of a 1.95 M NaOH M NaOH solution and excess NiCl2NiCl2?arrow_forwardWalter White requires a sulfuric acid (Strong acid) solution to use in a very important synthetic reaction. He finds a bottle labeled H2SO4, but by some strange turn of events, he cannot remember the concentration. He decides to perform a titration to determine the concentration of the H2SO4. He pours 22.20 mL of his unknown sulfuric acid solution into a flask and finds that it requires 29.50 mL of 0.57 M KOH (strong base) to reach the endpoint. What is the concentration of Walter White's sulfuric acid solution? (Enter your numeric answer only, do not use scientific notation, enter 2 decimal places with your answer) For reference: KOH = 56.106g/mol H2SO4= 98.079g/mol Avagadro's number 6.022x1023arrow_forwardHow many mL of 0.815 M KI solution are required to react with 50.0 mL of .259 M Pb(NO3)2 solution to form lead(II) iodide?arrow_forward
- A 185.0 mL sample of 1.200 M Pb(NO₃)₂ is mixed with 52.50 mL of 1.500 M NaCl, and the PbCl₂ precipitate is filtered from the solution. Then 200.0 mL of 3.000 M NaBr is added to the remaining solution, and the PbBr₂ precipitate is also collected and dried. What is the mass (in grams) of the PbBr₂ precipitate, assuming the yield in each precipitation step is 100%?arrow_forwardWhat volume of water is needed to prepare 500 mL of a 0.25 M Ca(NO3)2 solution from a stock solution of 5.0 M Ca(NO3)2?arrow_forwarddetermine the mass of CuS formed when 100.mL of 0.100M H2S and 50.0ml of 0.150M Cu(C2H3O2)2 are reacted. What is the concentration of C2H3O2 in the resulting solution?arrow_forward
- What volume of 0.100 M Na3PO4 is required to precipitate all the lead(II) ions from 350.0 mL of 0.250 M Pb(NO3)2 ?arrow_forward5.00 mL of acetic acid required 37.28 mL of 0.0999 M NaOH to be neutralized in a titration. Calculate the molarity of vinegar.arrow_forwardIt takes 28.61 mL of 0.216 M sodium hydroxide solution to titrate 10.00mL of sulfuric acid solution. What is the molarity of the sulfuric acid?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY