
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the name of the product that will be formed? (look at the picture to be able to answer)
a) ethyl butanal
b) butyl ethanoate
c) butyldietanone
d) acid anhydride
e) ethyl butanoate
f) None of the answers are correct.
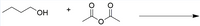
Transcribed Image Text:ОН
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- It was wrong can someone helparrow_forwardQuestion 2 of 2 Submit Alcohols undergo dehydration reactions in the presence of an acid catalyst. Which of the following compounds yields only a single alkene product upon dehydration? A) 1-methylcyclohexanol B) 1-butanol C) 2-butanol D) 2-hexanol E) 3-hexanolarrow_forwardComplete the following reaction, and answer the questions below: OH (NaoH) a) What s the name of this reaction? b) OH in the above reaction is a base or a nucleophile ?arrow_forward
- What ending should each of the molecules below have? a) Alcohols b) Aldehydes c) Carboxylic acids d) Aldehyde add speaker notesarrow_forwardOH ** What is the product of the reaction shown below? H₂CrO3 A) propane B) propanal C) propene D) propanoic acid E) propynearrow_forwardSelf Study/Carboxylic Acids/Synthesis/Reactions Name: 1. Show how you will accomplish the following synthesis of carboxylic acids. You can use any necessary reagents. a) 3-hexene b) 2-butenal propanoic acid 2-butenoic acid 2. What are the products of the following reactions used to synthesize carboxylic acids. a) 1) NaCN CH2Br 2) H₂O* Na2Cr2O7, H2SO4 b) CH₂OH KMnO4, H₂O -CH2OH c) A 3. What are the reagents used or products made in the following transformations? a) Hexanoic acid hexanal CH2OH COOH b) CH2COOH CH2CONHCH 3 c) Dr. Gupta/Carboxylic Acids-Synthesis Reactions-SS/Page 31arrow_forward
- can I get an explanation on how to do these? Thank youarrow_forwardSelect all of the functional groups that contain rings. ester aldehyde aromatic ring alkane phenol amide alkene thiol ether amine ketone alcohol carboxylic acid cycloalkane Those were the options and I tried many different ones so please provide the accurate ones. I already tried alcohol, aromatic ring, amine, and ether but it was wrong I tried the 3 without aromatic ring and it was also wrong. I tried 6 different waysarrow_forwardWhich is a correct name for this compound? NH₂ A) 2-nitrotoluene B) 2-aminotoluene C) 2-ethylnitrobenzene D) 2-ethylanilinearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY