
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
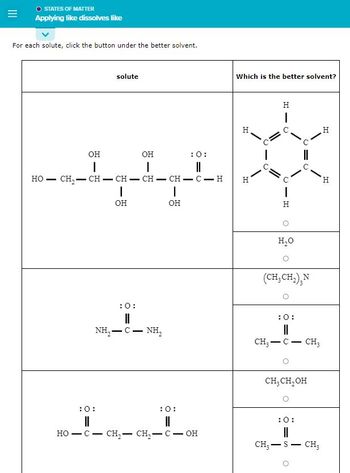
Transcribed Image Text:O STATES OF MATTER
Applying like dissolves like
For each solute, click the button under the better solvent.
HO
-
OH
|
solute
OH
I
:O:
II
:0:
11
NH,—C — NH,
CH₂CH=CH-CH-CH- C-H
I
OH
:0:
||
I
OH
= 0
:0:
||
HO-C- CH₂ - CH₂ - C - OH
Which is the better solvent?
H
H
C
H
I
O
I
H
H₂O
C=C
(CH₂CH₂), N
:0:
||
CH₂ - C - CH3
CH₂ CH₂OH
:0:
||
CH₂-S - CH3
O
H
H
Expert Solution
arrow_forward
Step 1 Theory
Like dissolves like.
Polar compounds are soluble in polar solvents and non-polar compounds are soluble in non-polar solvents.
Polar compound are those which have bonds between atoms of different electronegativity.
Non-polar compounds are those which have bonds between atoms of same electronegativity or nearly same electronegativity.
Protic solvents are those in which H bonded to an electronegative atoms like F,N and O.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Molecule Type Boiling point (°C) CH3CH2CH3 Alkane -42 CH3CHO Aldehyde +21 CH3CH2OH Alcohol +78 i. Why is the boiling point of the aldehyde greater than that of the alkane?ii. Why is the boiling point of alcohol the highest?iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forwardPlease provide only typed answer solution no handwritten solution allowedarrow_forwardGases, Liquids, and Solids Applying like dissolves like For each solute, click the button under the better solvent. H H solute Which is the better solvent? CC₁₁ CaCl2 CH₂OH 0 H C H H H₂O H 10: NH,C — NH, H H CH3(CH2)CH3 O CH, (CH₂),CH, H :0: || C-C-OH H X H 13arrow_forward
- 1. A 4 M acetic acid (CH3COOH) solution lowers the freezing point by -8oC; a 4 M KF solution yields a -15oC freezing point depression. What can account for this difference? 2. Based on what you have studied about intermolecular forces, predict which of the following liquid has the highest boiling point and why. All three compounds have comparable molecular weights. Butane, C4H10 b. Propanol, C3H7OH c. Acetone, CH3COCH3arrow_forwardFor each solute, click the button under the better solvent. solute :0: NH,—C — NH, NaC1 CH₂(CH₂) CH3 Which is the better solvent? H H CH₂(CH₂), CH₂ H I H :O: || C-C-OH CH, (CH₂) OH CH, OH O H₂O O CCI H Oarrow_forwardHydrogen bond can form among water molecules, proteins and nucleic acids. For a hydrogen bond, there is a "donor" and an "acceptor". Below which is NOT a possible combination of donor/acceptor for a hydrogen bond? O [C-H-0] O [O-H----:0] O N-H-----0] [N-H-----N]arrow_forward
- 40. Which of the following is not a polar protic solvent? :0: H | || H :0: H I || | Н-с-с-с-н н-с-ӧ-н н-ӧ-н н-с-с-ӧ-н .. H H A Darrow_forwardArrange the following in terms of increasing water solubility in section II and dipole moments in section III using the less than (<) inequality.arrow_forwardBelow is the Lewis structure of cyclohexane (C6H12): нн H \/ Н н-с н-с Н D 58 О нн О Which of the following is/are more soluble in this solvent than in water? D BaCl2 H₁ H C=C H C-H нн н-с-с-н н-о: н нӧ С-Н н Н Н с-с-н нarrow_forward
- In your own words, explain why water is known as the "universal solvent." Answer in complete sentences. H Normal BIUS ED ==== X₂ X² Ω Enter your answer here ΞΞΑ Α Tx GOarrow_forward5 L山 When 1.06 g of a certain molecular compound X are dissolved in 85.0 g of dibenzyl ether ((C,H,CH,),0), the freezing point of the solution is measured to be 0.9°C. Calculate the molar mass of X. If you need any additional information on dibenzyl ether, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to 1 significant digit. 自 OL Check Save For Later Submit Assignment O2022 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center Accessibility EPIC 中Xe MacBook Pro F2 F3 F4 F7 F8 F10 6-5 #3 3. 2 $ 一 8 dele R. A C. W alt alt option 10 command option 1Q15arrow_forwardExplaining Vapor Pressure in Terms of IMFs Given the shown structural formulas for these four compounds, explain their relative vapor pressures in terms of types and extents of IMFs: OH I H₂C-CH₂ ethanol HO OH | | H₂C-CH₂ ethylene glycol Solution CH₂CH₂OH: H-bonding & LDF. CH₂OHCH₂OH: Stronger H-bonding than ethanol (also LDF), and higher MM than H₂O. More viscous than ethanol, thus quite a low P vap. CH3-CH₂-0-CH₂ CH3 and H-O-H diethyl ether water C4H₁0O: Dispersion forces AND dipole-dipole. Diethylether is quite a volatile substance, with quite a high Pvap: vap• H₂O: H-bonding & LDF. Although the molecule with the LOWEST Molar Mass, it has Of the choices above, ONLY ethylene glycol has a LOWER Pvap- a low Pvap. LOWEST Pvap HIGHEST Pvaparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY