
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Draw an ionic bond between Asp and Arg side chains.
Explain your answer thoroughly. This question requires drawing on pen and pencil please.
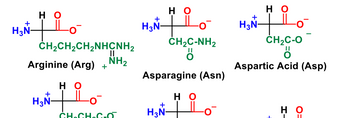
Transcribed Image Text:HO
HO
+
+
H3N-
CH2CH2CH2NHCNH2
CH2C-NH2
+
HO
найшо
CH2C-O
Arginine (Arg) +
NH2
Aspartic Acid (Asp)
Asparagine (Asn)
HO
+
HO
H3N-
+
CH. CH C o
H3N-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Subjecting this peptide to trypsin would result in L-M-F-V-W-E-Q-A-P-S-P O A. Lys and Met-Phe-Val-Trp-Glu-Gln-Arg and Pro-Ser-Pro OB, Leu-Met-Phe-Val-Trp-Glu-Gin-Ala-Pro-Ser-Pro Leu and Met-Phe-Val-Trp-Glu-Gln-Arg-Pro-Ser-Pro OC. Op. Lys and Met-Phe-Val-Trp-Glu-Gln-Ala-Pro-Ser-Pro Leu and Met-Phe-Val-Trp-Glu-Gin-Arg-Pro-Ser-Pro OE.arrow_forwardExplain the Titrimetric profile of Lysine in figure 2.arrow_forwardAmino sequence: Gln-Glu-Val-Leu-Ile-Arg-Leu-Phe-Lys-Gly-His-Pro-Glu-Thr-Leu-Glu-Lys-Phe-Asp-Lys-Phe-Lys-His-Leu-Lys-Ser-Glu-Asp-Glu-Met-Lys-Ala-Ser-Glu-Asp-Leu-Lys A) List the fragments generated with trypsin: B) List the fragments generated with chymotrypsin (5 pts) (assume reaction conditions are used to maximize cutting to the C-terminal size of only the 3 aromatic amino acids.)arrow_forward
- Explain the Titration profile of Lysinearrow_forwardFirst letter U C A UUU UU JUC UUA UUG CUU CUC CUA CUG AUU AUC AUA AUG GUU GUC GUA GUG ] Phenylalanine UCU (Phe) UCC UCA UCG Leucine (Leu) Leucine (Leu) Isoleucine (Ile) Methionine (Met) Valine (Val) CCU CCC CCA CCG b) lysine c) methionine d) tryptophan ACU ACC ACA ACG GCU GCC GCA GCG C Second letter Serine (Ser) Proline (Pro) Threonine (Thr) Alanine (Ala) UAU UAC UAA UAG CAU CAC CAA CAG AAU AAC AAA AAG GAU GAC GAA GAG ] Tyrosine (Tyr) Stop Stop Histidine (His) Glutamine (Gln) Asparagine (Asn) Lysine (Lys) Aspartic acid (Asp) Glutamic acid (Glu) UGU UGC UGA Stop UGG Tryptophan (Trp) CGU CGC CGA CGG AGU AGC AGA AGG GGU GGC GGA GGG ] 1. List the mRNA base codons for the amino acids listed below. 2. a) aspartate: Cysteine (Cys) Serine (Ser) U C Arginine (Arg) A G Arginine (Arg) U C Glycine (Gly) A G U C A G U MCAG Аarrow_forwardI don't understand how to do this problemarrow_forward
- The primary structure of b-endorphin, a peptide containing 31 amino acids synthesized by the body to control pain, is shown here: Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr- Leu-Phe-Lys-Asn-Ala-Ile-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu What fragments are obtained as a result of treatment withcyanogen bromide?arrow_forwardThe primary structure of b-endorphin, a peptide containing 31 amino acids synthesized by the body to control pain, is shown here: Tyr-Gly-Gly-Phe-Met-Thr-Ser-Glu-Lys-Ser-Gln-Thr-Pro-Leu-Val-Thr- Leu-Phe-Lys-Asn-Ala-Ile-Ile-Lys-Asn-Ala-Tyr-Lys-Lys-Gly-Glu What fragments are obtained as a result of treatment with trypsin?arrow_forwardTranslate the following DNA sequence into amino acids 5'ATAGTACCGCAAATTTATCGCT3 O met-ala-phe-lys-stop O met-ala-phe-lys- met-tyr-his-gly-val-stop-met-gly O met-ala-ser-gly-thr-stop O tyr-his gly-val-stop-met-ly O ala-phe-lys stoparrow_forward
- Calculate the pl of the following Peptide NH3*-Ala-Glu-His-Leu-COOHarrow_forwardConsider the following two peptides: I. N-Pro-Pro - Glu - Glu - Tyr - His - Cys - Ala - Glu - Gln - Lys - Leu - Ser - Ser - Phe-Leu- Thr - C II. N-Pro-Pro - Lys - Arg - Gly - Tyr - His - Gly - Glu - Asp - Glu - Asp - Glu - Ser - Gly-Phe- Tyr-C Give three reasons why_peptide I is more likely to form an alpha helix in aqueous solution at pH 7.0. Your reasons may include why_peptide Il is less likely to form an alpha helixarrow_forwardIdentify the following amino acids and their following side chain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON