
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Are these configurations correct?
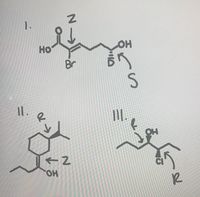
Transcribed Image Text:но
HO
Br
II.
HO.
R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1carrow_forwardGive the electron configurations for the ions Lig* and Liz in molecular orbital terms. Compare the Li-Li bond order in these ions with the bond order in Li2. Electron configuration for Lig + Electron configuration for Liz Bond order of Liz is than that of either of its ions. Drag and drop your selection from the following list to complete the answer: 1 2 1 2 16) (02)² (019) (oʻ1s)" (020)" * * (01s) (o 1s (01s) (o 1s O 2s O 2s O 1sarrow_forwardWhat are all of the secondary force that influence this compound NH3arrow_forward
- From the table of data below, calculate all of the interatomic equilibrium potential energies responsible for the molecule, methanol (CH3OH). Molecule/Radical/Atom CH3OH CH₂OH CH3O CH3 OH Н Enthalpy of Formation / Kcal mol¹ @ 298.15 K -48.1 -2.0 4.1 35.1 9.0 52.1arrow_forwardWhich of the atoms in this molecule are in the same plane? A f e I I H C H | a C=O g H a and b B a, b, and c C a, b, c, and d D a, b, c, d, and e E a, b, c, d, e, f, and garrow_forwardA benzene molecule can be modeled as six carbon atoms arrangedin a regular hexagon in a plane. At each carbon atom, one of threeradicals NH2, COOH, or OH can be attached. How many suchcompounds are possible? (Make no distinction between single anddouble bonds between the atoms.)arrow_forward
- When considering molecular orbital diagrams of homonuclear diatomic molecules, explain why atomic orbital energies are the same. How will this differ for heteronuclear diatomic molecules?arrow_forwardPlease don't provide handwritten solution ....arrow_forwardWhat is the deltaG f for NO2(g) at 25C? NO2(g) + N2O(g) <-> 3 NO(g) delta Hf kJ/mol 33.2 81.6 91.3 S J/mol K 240.1. 220.0 210.8 delta Gf kJ/mol ? 103.7 87.6 (answer in kJ, please)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY