
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Draw a complete stepwise mechanism for the acid-catalyzed hydration of the
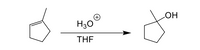
Transcribed Image Text:### Hydration of Methylcyclopentene
This diagram illustrates the acid-catalyzed hydration reaction of methylcyclopentene in the presence of hydronium ion (\( \text{H}_3\text{O}^+ \)) and tetrahydrofuran (THF) as solvent.
#### Reactants:
- **Methylcyclopentene**: This is the starting alkene with a double bond and a methyl group attached to a five-membered ring.
#### Reaction Conditions:
- **Hydronium Ion (\( \text{H}_3\text{O}^+ \))**: Acts as an acidic catalyst to facilitate the reaction.
- **THF (Tetrahydrofuran)**: Serves as the solvent to dissolve reactants and products, providing a medium for the reaction.
#### Product:
- **Alcohol**: The alkene is converted into an alcohol, with the OH group added to the former alkene carbon.
This transformation involves the addition of water across the double bond. The result is a more substituted alcohol due to the stability of the carbocation intermediate formed during the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a curved arrow mechanism that explains the formation of the given organic product in the following reaction. You may need to re-draw structures to show bonds or lone pairs. : ÖH H₂SO4 to 0 :0 2 + 10 And/Remove step Xarrow_forwardUsing Curved Arrow Formalism, draw the reaction of 1-methylcyclopentanol treated with aqueous sulfuric acid and identify the rate determining step. If more than one product is formed, identify which is major, minor, very minor, etc. Thank you for the help with this question. I appreciate it.arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron- pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. Drawing A no NO O NaⒸ CH₂ Subme PEarrow_forward
- Draw a curved arrow mechanism for the substitution reaction that will occur with the alkyl halide and nucleophile below, adding steps as necessary. Be sure to include all nonbonding electrons and all nonzero formal charges, and show all species that react or are formed in this reaction.arrow_forwardComplete the mechanism for this SN1 reaction by completing the given structures and adding the missing curved arrow notation. Do not delete or add entire structures, and be sure to include lone pair electrons and nonzero formal charges where appropriate. Kok H -H2O See Periodic Table :Br: :: i Complete the organic structure resulting from the arrows in step 1. Provide the missing curved arrow notation.arrow_forward(b) Show the mechanism of this reaction using proper arrow push notation. You must show all intermediates, formal charges, and necessary arrows. CH3 CH3 CH3 H20 H2SO4arrow_forward
- Draw a curved arrow mechanism for Step 1 of this reaction, showing the formation of the intermediate(s), including stereochemistry. Show all intermediate structures for this step.arrow_forward1. Take a close look at the following reactions. (a) Draw the product in the box below. (b) Provide the mechanism in the space below the reaction scheme. H₂O, H₂SO4arrow_forwardDraw the lewis structure for 2, 3, 3-trimethyl-1 - butene. Then, show the full curved arrow mechanisms when the alkene is Acid - catalyzed hydrated, reacted with water with a trace of acid catalyst like sulfuric acid H2SO4, WITHOUT a methyl shift.arrow_forward
- Draw the product of the E2 reaction shown below. Include the correct stereochemistry. Use a dash or wedge bond to indicate relative stereochemistry on asymmetric centers, where applicable. Ignore any inorganic byproducts. CI Strong Basearrow_forwardPlease don't provide handwritten solution .....arrow_forwardAcyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. H3C. ... NH2 HCI/H₂O reflux • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Na+, I, in your answer. • In cases where there is more than one answer, just draw one. + 4 n [ ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY