
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Propose a mechanism for the reaction of H2O2 with your cyalume.
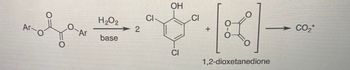
Transcribed Image Text:The image depicts a chemical reaction involving the conversion of an aryl dioxetanedione compound. The reaction is initiated in the presence of hydrogen peroxide (H₂O₂) and a base.
- **Reactant**: The starting material is an aryl-substituted dioxetanedione.
- **Reagents**: Hydrogen peroxide (H₂O₂) and a base.
- **Products**: The products include two equivalents of 2,4,6-trichlorophenol, 1,2-dioxetanedione, and carbon dioxide (CO₂) emitted with a positive charge.
This reaction illustrates a decomposition pathway yielding carbon dioxide gas as one of the products.
**Diagrams or Graphs**: The image provides structural formulas indicating the molecular arrangement of atoms in the reactants and products. The arrow shows the direction of the transformation and indicates the conditions required for the reaction (H₂O₂ and base).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show the reaction mechanism for the intramolecular openings of epoxides under basic or acidic conditions OPER OH OH H₂O* OH HO OH farrow_forwardDraw the products of the reaction sequence shown below. Ignore inorganic byproducts. H3O+ heat Drawing Qarrow_forwardThe following reactants will undergo a Robinson annulation reaction in the presence of base (KOH). Draw the expected organic product. Do not draw inorganic byproducts. H,C. H2O, KOHarrow_forward
- What reaction took place in the reaction below? CHẠCH, CH; CH,(CH),CH; CH{CH,),C-OCH; CHẠCH)A CH; CHỊCHCo H0 НОСН CH/CH),CH, CH, (CH),C-C HOCH, HH O reduction (hydrogenation) O amide synthesis O acetal hydrolysis O ester synthesis O acid base O dehydration O thioester synthesis O phosphate ester synthesis O hydration O ester hydrolysis O amide hydrolysis O oxidation O acetal synthesisarrow_forwardHow do I make a mechanism for this reactionarrow_forwardDraw the product of the reactionarrow_forward
- H₂C H H₂C H + + LIAIH [0] + [O] 7 Ethylbenzoate Give mechanism Acetic benzoic anhydride Give mechanism Benzylberarrow_forwardPropose a mechanism for this reaction.arrow_forward2. Draw a detailed mechanism for the following reactions. You do not need to draw arrow steps for the hydrogenation reaction. Ph 1. NaOH 2. CH31 3. H₂ Pd/C 4. H30* HBr en Ph^ Brarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY