
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
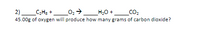
Transcribed Image Text:2)_C;H3 +_02→__H;0 + __CO2
45.00g of oxygen will produce how many grams of carbon dioxide?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ✓ 个 < app.101edu.co STARTING AMOUNT X How many grams of oxygen gas are produced when 2.43 x 104 g of KCIO, are completely reacted according to the following chemical equation: 3.5 1 ADD FACTOR * ( ) 32.00 9.52 x 10-5 g KCIO3 2 KCIO3(s) → 1.90 x 10-4 Question 31 of 38 2 mol KCIO3 O 3 2 KCI(S) + 3 M 74.55 mol KCI ANSWER 31 1.98 x 10-6 5.95 x 10-6 mol O₂ O₂(g) 2.43 x 104 6.022 x 10²3 g 0₂ by RESET 2 2.97 x 10-6 122.55 g KCI Feb 23 7:11 Oarrow_forwardWhat is the minimum mass of oxygen, in grams, needed to burn 30.3 g of C3H6O? Give your answer to three significant figures. The unbalanced chemical equation is: Molar masses, in g mol-¹: H, 1.008 Number Units C3H6O + O₂ C, 12.01 CO₂ + H₂O O, 16.00arrow_forwardHow many grams of CO, are produced when 23 grams of C,H,OH . are combusted?arrow_forward
- A compound has the composition: carbon 37.21%; hydrogen 7.83%; and chlorine 54.96%. If its MW is 385 g/mol, determine (a) the empirical formula (b) the molecular formula.arrow_forwardHow many grams of O2(g)O2(g) are needed to completely burn 80.8 g C3H8(g)?arrow_forward14. The fertilizer ammonium sulfate [(NH,),SO4] is prepared by the reaction between ammonia (NH3) and sulfuric acid: 2NH;(g) + H,SO,(aq) --->(NH,),SO,(aq) How many kilograms of NHz are needed to produce 1.00 x 10° kg of (NH,),SO,?arrow_forward
- Name: 14. The following reaction occurs and 9.81 x 1025 CO, molecules are produced. C6H12O6 (s) + O₂(g) → CO₂(g) + H₂O(1) How many kilograms of O₂ is required? © 2021 F'21/S'22 NetID: Chemistry I A: 5.21 WORKSH 83arrow_forward2H2 + O2 --> 2 H2O How many moles of oxygen is required to produce 19.7 moles of water?arrow_forwardYou have 22.2 g of the compound C2Hx, where x is the maximum number of hydrogens that can fit around two carbon atoms and still create a stable Lewis dot structure. If you allow all of this sample to react with excess oxygen and then allow the products to cool to room temperature, how many moles of liquid product will you have?arrow_forward
- Request They Both Die at t... ula Enchanted Wa... Question 6 of 20 Balance the following chemical equation (if necessary): Sio,(s) + C(s) → Si(s) + CO(g) 04- 2. 3+ O4+ 1 6. 7 8. 9. Os 6. O9 (s) (1) (g) (aq) C Si Reset • x HO Delete MacBook Air 000 F3 F4 F5 @ %23 2$ % 2 3 5 6. 8 9. W E 5 4- 3. 2. 14arrow_forward10. Using the following reaction 2KCIO; -2KCI +302 If 10g of oxygen were produced, what mass of KCIO, (GFM = 122.6g/mol) was added? %3D Number unitarrow_forwardIf you wanted to produce 4 moles of SO3 gas, how many moles of SO2 would you need to react? (Assume an unlimited supply of O2 gas.) 2 SO₂ (g) + O₂(g) →2 SO, (g)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY