
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the major product/s for each of the reactions shown below.
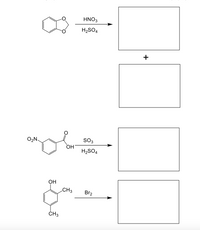
Transcribed Image Text:HNO3
H2SO4
O2N.
SO3
OH
H2SO4
ОН
CH3
Br2
ČH3
+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. Provide the product that will result for the following reaction and provide the complete mechanism. CH,N2 НО 오arrow_forwardDraw the major substitution products you would expect for the reaction shown below. If substitution would not occur at a significant rate under these conditions, check the box underneath the drawing area instead. Be sure you use wedge and dash bonds where necessary, for example to distinguish between major products. Note for advanced students: you can assume that the reaction mixture is heated mildly, somewhat above room temperature, but strong heat or reflux is not used. Br O Substitution will not occur at a significant rate. : CN + X Click and drag to start drawing a structure.arrow_forwardConsider the following reactants: Br Would elimination take place at a significant rate between these reactants? If you said elimination would take place, draw the major products in the upper drawing area. If you said elimination would take place, also draw the complete mechanism for one of the major products in the lower drawing area. If there is more than one major product, you may draw the mechanism that leads to any of them. Major Products: Oyes поarrow_forward
- Propose a mechanism for the reaction shown here, which takes place under conditions that favor anarrow_forwardIf H* is eliminated from the carbocation shown here in an electrophile elimination step, then three possible constitutional isomers can form. Draw the mechanism for the formation of all three of those products. H2O + ?arrow_forwardE. Show the products in the following reactions. If more than one product is possible, label the major product. H3CO O NO₂ SO3, H₂SO4 SO3H NO₂ Br₂, FeBr3 CH3I, AIC13 HNO3, H₂SO4arrow_forward
- Please propose a mechanism for the reaction which takes place under conditions that favor an SN1 reaction.arrow_forwardDraw all the products in circle the major product of the following E1 reaction. What is the rate expression( rate equation)arrow_forward1) A student measures 7.45 cm³ of chloroform using a glass syringe. Given that the density of chloroform is 1.48 g/mL, and the molar mass is 119.38 g/mol, how many moles of chloroform did the student measure?arrow_forward
- Draw the major organic product of the reaction shown below. OH NaNH2arrow_forwardDetermine the step by step mechanism of the major product of the following reaction below. Furthermore, determine all-of the product for the following reaction and circle the major product and explain. Clz hvarrow_forwardDraw a detailed arrow pushing mechanism for the following reaction. In the rate-determining step unimolecular (SN1) or bimolecular (SN2)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY