
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
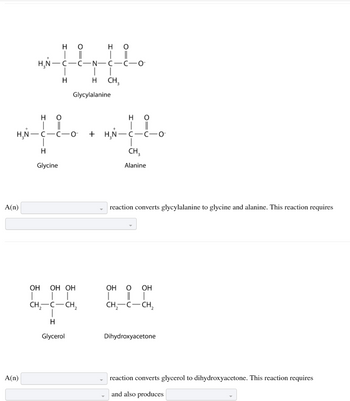
Transcribed Image Text:A(n)
A(n)
H₂N-
H
|
O
||
H₂N -CICIO
_
HO
|||
H
Glycine
OH OH OH
T
CH₂-C-CH₂
H
Glycerol
H O
H H CH₂
Glycylalanine
C-N-C
+ H₂N-
C-O
HO
T ||
CICIO
CH₂
Alanine
reaction converts glycylalanine to glycine and alanine. This reaction requires
OH O OH
I I
CH₂-C-CH₂
Dihydroxyacetone
reaction converts glycerol to dihydroxyacetone. This reaction requires
and also produces
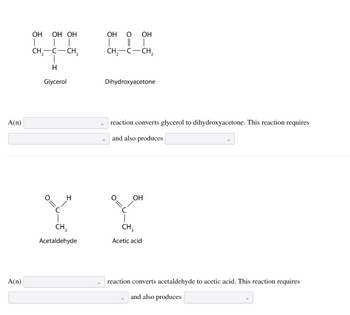
Transcribed Image Text:A(n)
A(n)
OH OH OH
T | |
CH₂-C-CH₂
T
H
Glycerol
-I
O=
с
H
CH₂
Acetaldehyde
OH O OH
|
CH₂-C-CH₂
Dihydroxyacetone
reaction converts glycerol to dihydroxyacetone. This reaction requires
and also produces
O=
C
OH
CH3
Acetic acid
reaction converts acetaldehyde to acetic acid. This reaction requires
and also produces
Expert Solution
arrow_forward
Step 1
1) Glycylalanine is a protein formed by the combination of glycine and alanine. Thus, glycylalnine has a peptide bond that can be hydrolyzed to give back its constituent amino acids- glycine and alanine. This process is called hydrolysis of peptide bonds and thus needs the presence of an enzyme called protease or peptidase.
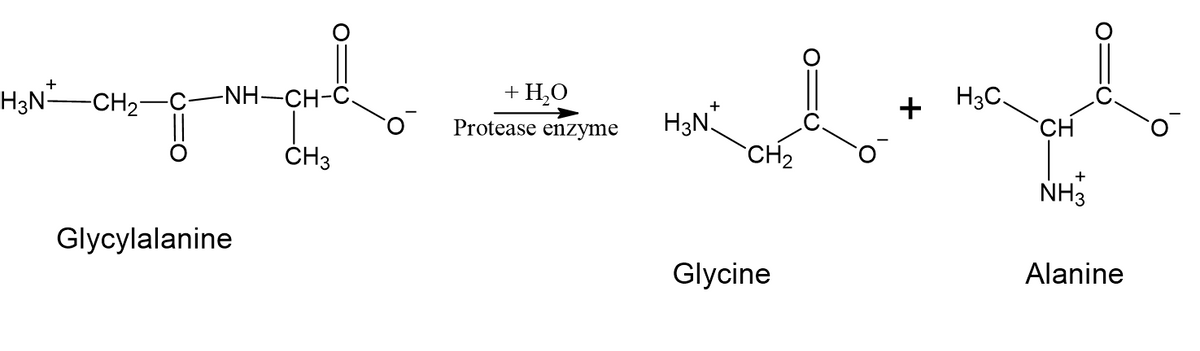
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How is THC-O acetate synthesized from delta-9 THC? Please draw out the reaction mechanism and cite your sources with an explanation for each step.arrow_forwardWhen an optically active D-aldopentose was subjected to Kiliani-Fischer synthesis, followed by NaBH4/H2O, it produced a mixture of an optically active and an optically inactive alditol. When the same D-aldopentose was subjected to Wohl degradation followed by HNO3 it produced an optically inactive aldaric acid. Provide the structure of this D-aldopentose. Н- НО- н- H -ОН НО -H Н- -Н CH₂OH 1 A) 1 B) II C) III осн H D) IV НО осн -H Н- -OH H- H HỌ CH₂OH П -OH H -OH H Н -H CH₂OH Ш _H -OH HO -OH H- -OH H- осн -H -OH CH₂OH IV -OH CH₂OH Varrow_forward22arrow_forward
- Propose at least three methods to convert C6H5CH2CH2Br to C6H5CH2CH3.arrow_forwardXylulose-5-phosphate is an intermediate product of metabolism with two stereogenic centers, as shown in the given skeletal structure. It has 1S, 2R configuration at its stereogenic centers. Redraw xylulose-5-phosphate, showing the correct configuration at each of the stereogenic centers. OH fex 2 OH OH OH OH Click and drag to start drawing a structure. X Ś èarrow_forwardGive the products of periodic acid oxidation of each of the following. How many moles of reagent will be consumed per mole of substrate in each case? (a) D-Arabinose (b) D-Ribose (c) Methyl ẞ-D-glucopyranoside (d) CH₂OH HO H O OCH 3 H OH H H H OHarrow_forward
- Draw structures for A, B, and C in the following reaction sequence and identify the process that converts B to C.arrow_forwardH-C -OH CH₂OPO₂²- 3-phosphoglycerate ATP ADP Arrow-pushing Instructions AC XT H-C-OH :O: CH₂OPO3²- =C² H-C-OH -O OPO3² Enz-SH 3 CH₂OPO3²- 1,3-bisphosphoglycerate ADP PO S-Enz H-C-OH CH₂OPO3²- One step in the gluconeogenesis pathway for the biosynthesis of glucose is the partial reduction of 3-phosphoglycerate to give glyceraldehyde 3-phosphate. The process occurs by phosphorylation with ATP to give 1,3-bisphosphoglycerate, reaction with a thiol group on the enzyme to give an enzyme-bound thioester, and reduction with NADH. Draw curved arrows to show the movement of electrons in this step of the reaction mechanism. [Enzyme-bound thioester] NADH/H* NAD*, Enz-SH H H-C-OH CH₂OPO3²- Glyceraldehyde 3-phosphate :Ö: H-C-OH CH₂OPO3²- ADP 29arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY