
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
How would you accomplish the following syntheses? You may use any specialized organic reagents, inorganic reagents and solvents that you need. Utilize the given starting materials.
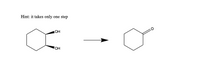
Transcribed Image Text:Hint: it takes only one step
CH
Expert Solution
arrow_forward
Step 1
A dehydration reaction is the reaction where water is eliminated from the reacting molecule, it is the reverse of a hydration reaction. Dehydrating agents commonly used are concentrated sulfuric acid and alumina.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the missing reagents for the following transformations. Multiple steps may be required. Br HO + En. HO Br OHarrow_forwardProvide reagents/conditions to accomplish the following syntheses. More than one step is required in some cases.arrow_forwardSuggest a step by step mechanism for this reaction.arrow_forward
- Synthesis: Show how you would carry out the following synthesis. Include the reagents you would need for each step and the intermediate products formed in each step. You may use any inorganic reagents you need and organic reagents with 6 or fewer carbons.arrow_forwardThe synthesis above used bromoethane, but acetylene is the only allowed source of carbon atoms. Using the reagents given, identify a synthetic route for the production of bromoethane from acetylene. H- Br The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as “EBF"). If there is more than one correct solution, provide just one answer. A В МeONa NaNH2 Н2, Pt D E F HBr H2, Lindlar's cat. Melarrow_forwardIF esize this compound by the → t-butyl ethyl ether Show the steps necessary to synthesize this compound by a rignard reaction. Start with an alkyl halide; after that you can add any organic or inorganic compound. → 1-hexanol Consider the following compounds:arrow_forward
- Provide the major organic product of the following reaction. + { A1C13arrow_forwardStarting from ethanol as the only organic reagent you have and use any other inorganic reagents to prepare:arrow_forward4. Which of the following reactions can be used to prepare 11-diphenyl-1-propanol? OH I. II. III. CH₂CH₂C снене-ось CH₂CH₂C -OCH3 PO 1. 2. H₂00 1. ? 2. H30Ⓡ MgBr MgBr 1. CH3CH₂ MgBr 2. H30arrow_forward
- Starting from the following alcohol and given the reagents listed below, design a reaction that would lead to the following NOTE: you may not need to use all of the reagents listed. Provide reasoning throughout.arrow_forwardUsing a series of reaction equations, outline how you would synthesize the following compounds from the reactants given. Show all reagents and necessary reaction conditions. You may use any reagents or solvents you cannot use starting materials other than those given. necessary, but a) 2,4-dimethyl-2-pentanol from 4-methylpentene and methyl bromide b) Heptanal from 1-heptene c) d) CH₂CH₂CH₂ CH2CH2CH2−O–C–CH3 from CH3 Ethylcyclohexane from CH3 -CH₂CH=CH₂ and tert-butyl alcohol aa CH₂Brarrow_forwardThe synthesis above used bromoethane, but acetylene is the only allowed source of carbon atoms. Using the reagents given, identify a synthetic route for the production of bromoethane from acetylene. The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagent(s) in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY