
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Fill in the missing reactant, reagent and products. Indicate stereochemistry if necessary. Unless otherwise specified, assume the reagents are in excess.
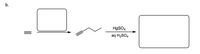
Transcribed Image Text:b.
HgSO4
aq H2SO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the IUPAC name for each compound. OH a. CH3CH(CH₂)4CH3 (select) OH (CH3CH₂)2CHCHCH₂CH3 (select) b. C. d. CH3 OH (select) (select) (select) OH (select) (select) (select)arrow_forward8) Show how the following conversions might be accomplished, including all reagents and intermediates. Br Y. OHarrow_forwardDraw the product(s) of the following reactions. CH3 H₂C-C-CEC-H OH 1. BH3/THF 2. H₂O₂/ aqueous NaOH • You do not have to consider stereochemistry. Separate multiple products using the + sign from the drop-down menu. • You do not have to explicitly draw H atoms. • If no reaction occurs, draw the organic starting material.arrow_forward
- ‘A’ compound works well with hard water. It is used for making shampoos & products for cleaning clothes. A is not 100% biodegradable and causes water pollution.‘B’ does not work well with hard water. It is 100% biodegradable and does not create water pollution. Identify A & B.arrow_forwardDraw the major product from this reaction. Use wedge and dash bonds to indicate relative stereochemistry where appropriate. Ignore acid byproducts. mCPBAarrow_forwardFill in the boxes with the starting material, reagent, synthetic intermediate, or product, as requested. 1. H9OAC2 , 2. NaBH4 H2SO4 heat excess HIarrow_forward
- These are synthesis questions. You need to show how the starting material can be converted into the product(s) shown. You may use any reactions we have learned. Show all the reagents you need. Show each molecule synthesized along the way and be sure to pay attention to the regiochemistry and stereochemistry preferences for each reaction. If a racemic molecule is made along the way, you need to draw both enantiomers and label the mixture as "racemic".arrow_forwardDraw the major organic product(s) of the following reactions including stereochemistry when it is appropriate. ● CEC • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If no reaction occurs, draw the organic starting material. Separate multiple products using the + sign from the drop-down menu. MULL ? H₂O/H₂SO4/HgO ChemDoodleⓇ Sn [Farrow_forwardDraw the major organic product(s) of the following reactions including stereochemistry when it is appropriate. H20/ H,SO, / HgSO4 CH,CH2-CEC-CH, • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If no reaction occurs, draw the organic starting material. Separate multiple products using the + sign from the drop-down menu.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY