
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the balanced equation for this reaction (in lowest multiple integers)?
![---
**H₂(g) + I₂(s) → 2HI(g)**
1. **Mass of HI Produced**
Given an initial mass of 17.8 g H₂, an excess of I₂, and assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of HI produced by the reaction.
**Input:**
- Enter mass in grams (g): [Input box]
**Action:**
- Submit [Button]
2. **Mass of H₂ Consumed**
Determine the mass (g) of the reactant, H₂, consumed by the reaction.
**Input:**
- Enter mass in grams (g): [Input box]
**Action:**
- Submit [Button]
3. **Moles of H₂ Consumed**
The entire mass, 17.8 g, of the reactant H₂ is consumed by the reaction. Calculate the amount (mol) of H₂ in this mass.
**Molar mass = 2.02 g/mol**
**Input:**
- Enter amount in moles (mol): [Input box]
**Action:**
- Submit [Button]
4. **Moles of HI Formed**
Given that 8.8294 mol H₂ was consumed, calculate the amount of HI formed.
**Input:**
- Enter amount in moles (mol): [Input box]
**Action:**
- Submit [Button]
5. **Mass of HI Formed**
Complete consumption of H₂ yields 17.6587 mol of HI. Calculate the mass of this quantity of HI.
**Molar mass = 127.91 g/mol**
**Input:**
- Enter mass in grams (g): [Input box]
**Action:**
- Submit [Button]
---
This transcription elaborates on the chemical reaction process, guiding students through calculations involving mass and mole conversions within this specific chemical context.](https://content.bartleby.com/qna-images/question/41533dd6-be05-40da-a627-190380aa1e53/95ddd29b-1043-4154-8640-e5ad14007853/fy82z6k_thumbnail.png)
Transcribed Image Text:---
**H₂(g) + I₂(s) → 2HI(g)**
1. **Mass of HI Produced**
Given an initial mass of 17.8 g H₂, an excess of I₂, and assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of HI produced by the reaction.
**Input:**
- Enter mass in grams (g): [Input box]
**Action:**
- Submit [Button]
2. **Mass of H₂ Consumed**
Determine the mass (g) of the reactant, H₂, consumed by the reaction.
**Input:**
- Enter mass in grams (g): [Input box]
**Action:**
- Submit [Button]
3. **Moles of H₂ Consumed**
The entire mass, 17.8 g, of the reactant H₂ is consumed by the reaction. Calculate the amount (mol) of H₂ in this mass.
**Molar mass = 2.02 g/mol**
**Input:**
- Enter amount in moles (mol): [Input box]
**Action:**
- Submit [Button]
4. **Moles of HI Formed**
Given that 8.8294 mol H₂ was consumed, calculate the amount of HI formed.
**Input:**
- Enter amount in moles (mol): [Input box]
**Action:**
- Submit [Button]
5. **Mass of HI Formed**
Complete consumption of H₂ yields 17.6587 mol of HI. Calculate the mass of this quantity of HI.
**Molar mass = 127.91 g/mol**
**Input:**
- Enter mass in grams (g): [Input box]
**Action:**
- Submit [Button]
---
This transcription elaborates on the chemical reaction process, guiding students through calculations involving mass and mole conversions within this specific chemical context.
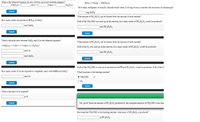
Transcribed Image Text:**Chemical Reaction Worksheets**
**1. Balancing Chemical Equations:**
- **Reaction:** \( \_\_\_ \text{MnO}_2(s) + \_\_\_ \text{Al}(s) \rightarrow \_\_\_ \text{Mn}(s) + \_\_\_ \text{Al}_2\text{O}_3(s) \)
- *Enter the balanced equation in lowest multiple integers.*
**2. Stoichiometry Calculations:**
a. **Calculating Moles of MnO₂:**
- *Question:* How many moles are present in 53.9 g of MnO₂?
- *Enter moles of MnO₂:*
b. **Mole Ratio Determination:**
- **Reaction:** \( 3 \text{MnO}_2(s) + 4 \text{Al}(s) \rightarrow 3 \text{Mn}(s) + 2 \text{Al}_2\text{O}_3(s) \)
- *Enter the mole ratio of Al to MnO₂:*
c. **Reacting Moles of Al:**
- *Question:* How many moles of Al are required to completely react with 0.620 mol MnO₂?
- *Enter moles of Al:*
d. **Mass of Al Required:**
- *Question:* What is the mass of Al required?
- *Enter mass of Al in grams:*
e. **Formation of Iron(III) Chloride:**
- **Reaction:** \( \text{Fe}(s) + \text{Cl}_2(g) \rightarrow \text{FeCl}_3(s) \)
- *Question:* How many milligrams of iron(III) chloride result when 20.00 mg of iron is reacted with excess chlorine gas?
- *Enter mass in mg of FeCl₃:*
**3. Limiting Reagent Analysis:**
a. **Amount of Acetic Acid Formed:**
- *Question:* What amount of HC₂H₃O₂ can be formed from the amount of each reactant?
- If all of the CH₃CHO was used up in the reaction, how many moles of HC₂H₃O₂ would be produced?
- *Enter moles of HC₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write a balanced chemical equation based on the following description: liquid C₇H₈O is burned with oxygen gas to produce gaseous carbon dioxide and water vaporarrow_forwardExplain the term "limiting reactant". What is an ionic equation? Explain the meaning of "the reaction goes to less than 100% completion".arrow_forwardBalance the chemical equation. CS₂ + NH3 H₂S + NH4SCN Assume the coefficient of NHSCN is 1. What is the balanced equation? CS₂ + NH3→H₂S+NHSCNarrow_forward
- What type of reaction is represent by the equation: C,H12 + 902 → 6CO2 + 6H20arrow_forwardAsprin can be made in a laboratory by reacting acetic anhydride (C4H6O3) with salicylic acid (C7H6O3) to form asprin (C9H8O4) and acetic acid (C2H4O2). The balanced equation is: C4H6O3 + C7H6O3 ------> C9H8O4 + C2H4O2 In a laboratory synthesis, a student begins with 5.00 mL of acetic anhydride (density = 1.08 g/mL) and 2.08g of salicylic acid. Once the reaction is complete, the student collects 2.06g of asprin. Determine the percent yield for the reaction.arrow_forwardBalance the chemical equation below using the smallest possible whole number stoichiometric.coefficients. Na(s) + O₂(g) Na₂O₂ (s) Xarrow_forward
- Balance the chemical equation below using the smallest possible whole number stoichiometric coefficients. C(s) + H, (g) → C,H, (g)arrow_forwardCarborundum (silicon carbide), SiC, is a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, SiO2, with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. (a) Write the balanced equation for this reaction. (Omit states - of - matter from your answer. Use the lowest possible whole number coefficients.) chemPadHelp (b) Calculate how many kilograms of SiO2 are required to produce 3.14 kg of SIC.arrow_forwardAsprin can be made in the laboratory by reacting acetic anhydride (C4H6O3) with salicylic acid (C7H6O3) to form asprin (C9H8O4) and acetic acid (C2H4O2). The balanced equation is: C4H6O3 + C7H6O3 ------> C9H8O4 + C2H4O2 In a laboratory synthesis, a student begins with 5.00 mL of acetic anhydride (density = 1.08 g/mL) and 2.08g of salicylic acid. Once the reaction is complete, the student collects 2.06g of asprin. determine the theoretical yield of asprin for the reactionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY