
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
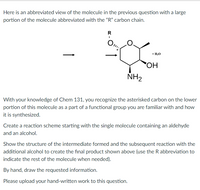
Transcribed Image Text:Here is an abbreviated view of the molecule in the previous question with a large
portion of the molecule abbreviated with the "R" carbon chain.
R
+ H,0
HO,
NH2
With your knowledge of Chem 131, you recognize the asterisked carbon on the lower
portion of this molecule as a part of a functional group you are familiar with and how
it is synthesized.
Create a reaction scheme starting with the single molecule containing an aldehyde
and an alcohol.
Show the structure of the intermediate formed and the subsequent reaction with the
additional alcohol to create the final product shown above (use the R abbreviation to
indicate the rest of the molecule when needed).
By hand, draw the requested information.
Please upload your hand-written work to this question.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 17) which of these 5 compounds is likely to smell BEST ?arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardIdentify (circle and name) all the functional groups in the following structures of the reactant and product. There may be functional groups that were not discussed in the video, so please use the functional groups reference sheet to assist you.arrow_forward
- How could one convert an aldehyde to a carboxylic acid? Group of answer choices- Reduce the aldehyde with hydrogen gas (H2) Oxidize the aldehyde using a mild oxidizing agent (such as permanganate). Combust the aldehyde with molecular oxygen. Use any of the above; each one would work OK.arrow_forwardPlease help with thisarrow_forwardNeed help with these reaction questions. Include all products please.arrow_forward
- The following chemical equation is for a substitution reaction, where the important atoms and groups involved have been highlighted for you: Hot. way + H HO O. H* ? + H-OH Use the information provided to predict the missing organic product and draw its structure in the drawing area below. Make sure to use the same skeletal ("line") style for the structure as the rest of the equation. Click and drag to start drawing a structure. X :0 S Omarrow_forwardGive the systematic (IUPAC) names for these molecules. Boononon cnolonongron. НаСНз CHОССH2СH2СНCHЗ CH3 phenyl propanoate |4-methyl pentane methanoat Incorrect. You mixed up the acyl and alkoxy portions of the molecule. Name the alkoxy part first, followed by the acyl part.arrow_forwardPls it says it’s wrongarrow_forward
- Q1 This question has two parts Part A and Part B. Part 1: Acetone can react with a base to give the enolate ion A, which can further react with another acetone molecule to give B. Upon quenching with water, B generates C, the final product of this reaction. :Base H20 A H3C H. H3C H. Acetone a) Draw the structure of A, B, and C b) Using curly arrows, draw the mechanism of the reaction between A and acetone to give compound B. What's the name of this reaction. - c) Draw the two resonance forms (A and D) of the enolate (A) generated by the deprotonation of the a-carbon on acetone and show the interconversion between the two using curly arrows: I Base H3C H A D H Acetone d) Knowing that the pka of the a-proton on acetone is 19, choose amongst the following bases those who will be able to remove the proton, given the pKa of their corresponding conjugated acids. H3C-C-H H H :Base H-Base + conjugate acid pka = 19 pКa 3 а, b, с, d a) Ammonia; pKa = 9.2 b) Sodium amide; pka = 38 c) Sodium…arrow_forwardWhich of the following molecules is an acetal (Image added)arrow_forward3.Complete the following Reaction. Include the IUPAC name of the product. [ OH + H₂C-CH₂CH₂-NH₂ a. H.S0₁ H50, +H₂C-OH- b. H,C—CH, + HO OH -CH₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY