
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![**Chemical Reaction Between Magnesium Nitride and Water**
When water is added to magnesium nitride and heated, ammonia gas is produced. The chemical equation for this reaction is:
\[
\text{Mg}_3\text{N}_2 (s) + 3 \text{H}_2\text{O} (l) \rightarrow 3 \text{MgO} (s) + 2 \text{NH}_3 (g)
\]
**Problem Statement:**
Calculate the volume (in mL) of \(\text{NH}_3\) produced from 1.82 g of \(\text{Mg}_3\text{N}_2\) at standard temperature and pressure (STP). Provide the answer with zero decimal places.](https://content.bartleby.com/qna-images/question/298ed52e-226c-4f85-994a-608c21d036d3/8e01205f-cc85-45ad-a5b0-8735113b84b6/k7j4j8_thumbnail.jpeg)
Transcribed Image Text:**Chemical Reaction Between Magnesium Nitride and Water**
When water is added to magnesium nitride and heated, ammonia gas is produced. The chemical equation for this reaction is:
\[
\text{Mg}_3\text{N}_2 (s) + 3 \text{H}_2\text{O} (l) \rightarrow 3 \text{MgO} (s) + 2 \text{NH}_3 (g)
\]
**Problem Statement:**
Calculate the volume (in mL) of \(\text{NH}_3\) produced from 1.82 g of \(\text{Mg}_3\text{N}_2\) at standard temperature and pressure (STP). Provide the answer with zero decimal places.
Expert Solution
arrow_forward
Step 1
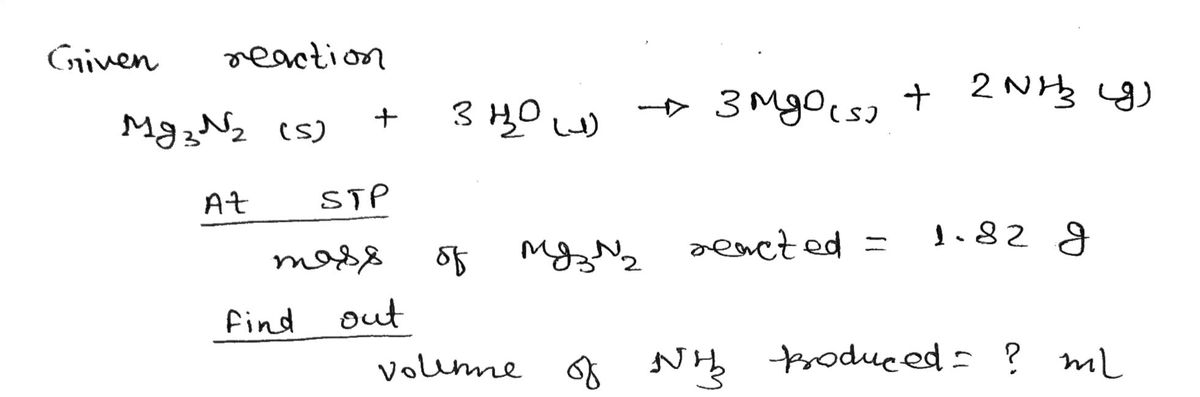
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The directions say: Do the following calculations and use the correct number of significant figures in your answers. Assume all numbers are the results of measurements.arrow_forwardA chemist adds 800.0 mL of a 50.0 g/dL calcium bromide (CaBr,) solution to a flask. Calculate the mass in kilograms of calcium bromide the chemist has added to the flask. Be sure your answer has the correct number of significant digits. O kgarrow_forwardCalculate the mass of ammonia (NH;) that contains a trillion (1.0 × 102) hydrogen atoms. Be sure your answer has a unit symbol if necessary, and round it to 2 significant digits. ?arrow_forward
- 1) The volume of a cylindrical object is 30.0 m3. What is the volume in in3?arrow_forwardwhich is in th7 place in 115.87arrow_forwardA 145.3 mL sample of carbon dioxide was heated to 301 K. If the volume of the carbon dioxide sample at 301 K is 394.6 mL, what was its temperature at 145.3 mL?arrow_forward
- Nickel crystallizes in a face-centered cubic lattice and has a density of 8.90 g/cm3. What is its atomic radius in picometers? MM of Ni is 58.69 g/mol. Express your answer in decimal notation rounded to three significant figures.arrow_forwardWhat temperature (in Kelvin) is a temperature of 51.67°C?arrow_forwardA 395.9 mL sample of carbon dioxide was heated to 323 K. If the volume of the carbon dioxide sample at 323 K is 645.2 mL, what was its temperature at 395.9 mL? Karrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY