
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
- Heat of combustion of gasoline is 50 MJ/kg. What mass of the gasoline is needed to release 10 MJ of heat?
/Give the answer in [g] /
Expert Solution
arrow_forward
Step 1
Given information:
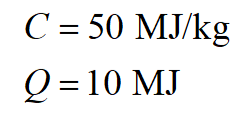
Here, C is the combustion of the gasoline (i.e., the heat released per kg of fuel) and Q is the heat released by the gasoline.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What heat is required to cause water of 250 g to be boiled. The initial temperature of water is 20°C? /Give the answer in [kJ]/arrow_forwardWhat is the magnitude of energy (in MJ) that must be removed to freeze 439 L of water with a density of 999.8 Kg/m3 that is already at 0˚C [round your final answer to one decimal place]? {latent heats of water: Lf = 33.5 × 104 J/kg, and Lv = 22.6 × 105 J/kg}arrow_forwardThe latent heat of fusion for ice is 3.34 x 105 J/kg at 0°C and atmospheric pressure. If the change in specific volume on melting is -9.05 x 10-5 m³/kg, (a) calculate the change of the melting temperature due to the change of pressure. (b) Estimate the freezing temperature of water at 200 m underneath the sea level. (c) Evaluate the importance of this natural property of water to the ecosystem under the lake.arrow_forward
- How much energy is required to completely melt 5.00 kg of gold which is initially at room temperature (20° C)? [Gold is often stored in 5.00 kg bars.Some physical properties of gold:specific heat capacity of solid gold -- 129 J/kg°Cfreezing/melting point -- 1063° Clatent heat of melting -- 6.44 x 104 J/kg]arrow_forwardWhat is the magnitude of energy (in MJ) that must be removed to freeze 466 L of water with a density of 999.8 Kg/m3 that is already at 0˚C [round your final answer to one decimal place]? {latent heats of water: Lf = 33.5 × 104 J/kg, and Lv = 22.6 × 105 J/kg}arrow_forwardA 4.09 kg silver ingot is taken from a furnace, where its temperature is 747 ∘C, and placed on a very large block of ice at 0.00 ∘C. Part A) Assuming that all the heat given up by the silver is used to melt the ice and that not all the ice melts, how much ice is melted? m= --------kgarrow_forward
- Hello, Can you please answer this question? The heat of combustion of gasoline is approximately 47 kJ /g . If a gasoline engine operated between 1500 K and 750 K, what is the maximum height that 5.0g of gasoline can lift an aircraft that weighs 400 kg?arrow_forwardA2] The figure shows a copper disk of radius r and thickness r, with a hole through its centre of radius a: Find an expression for the volume of the copper. b) Find an expression for the increase in temperature of the copper AT if it is supplied with an amount of heat Q at constant pressure. The density and specific heat (heat capacity per unit mass) of copper at constant pressure are PCu and ccu respectively. (You should treat these quantities as constants even though thermal expansion will slightly reduce the density, for example). c) of the disk AA/A when its temperature increases by AT, if the coefficient of linear expansion of copper is aCu- (change in dimension)", such as (Ar). Find an expression for the fractional increase in area of the top surface You should ignore terms of the orderarrow_forwardHow much heat is required to convert 5 kg of ice at -20C to steam at +100C? (relevant constants given below for convenience) Cice= 2.108x103 J/kg°C Cwater= 4.186x10³ J/kg°C L= 3.335x105 J/kg Ly= 2.260x106 J/kg O 2.303 × 106 J O 1.360 × 107 J O 1.485 × 107 J O 1.527 × 107 J O 1.570 × 107 Jarrow_forward
- How much heat (in joules) is required to raise the temperature of 24.4 kg of water from 19 ∘C to 88 ∘C? Express your answer using two significant figures.arrow_forwardAir at 21°C is blown over a hot pipe with a surface area of 3.61 m2 to dissipate 922 W of heat energy. What is the minimum convection heat transfer coefficient that will ensure that the temperature of the pipe surface is less than 45°C [round your final answer to two decimal places]?arrow_forwardThe temperature of an aluminum disk is increased by 250 ∘C. By what percentage does its volume increase?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON