
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Construct table that includes the carbon type and chemical shift for compound P.
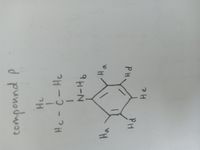
Transcribed Image Text:He
He
PH
PH
9H-N
1
Hc-C- Hc
componnd p
oun
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- Predict whether aqueous solutions of the following compounds are acidic, basic, or neutral. compound solution is...? acidic NH, Br basic 4 neutral acidic NaN O, basic neutral acidic Fe(C10.), basic neutral O OO O O Oarrow_forwardPlease give me the right answerarrow_forwardalso specify the integration valuesfor all Hs in each molecule.arrow_forward
- Nonearrow_forwardIr spectrum for cyclohexanol and the peaks on the graph identified with the board,strong,weak, and the groupsarrow_forwardFigure 3-9. 100 FUNSHINE 4800 001 Linti 4000 100 4900 3000 3000 3000 B 2008 2008 C 2008 HAVENUMER NAVENURIER-) NAVENUTEERIT CUST 1300 1900 1000 1000 1000 500 500arrow_forward
- Can you please help me with this ochem question?arrow_forwardhow does the chemical shift change when a carbon is connected to an electronegative atom via double bond compared to a single bond? Is the carbon signal for a carbon in a double bond with oxygen downfield or upfield?arrow_forwardfa) In the 400 MHz spectrum of a certain , separated by 4 ppm. The observed compound, there separated by 4 ppm. The observed coupling constant is 7 Hz. 6) What is this separation expressed in are two doublets Hz? f) In a 600-MHz instrument, what is the separation in Hz and in ppm?arrow_forward
- Solution please, asked two times before but getting wrong answer. Please need correct, otherwise will report.arrow_forwardSee attached picture. Question.) Below is the NMR spectra and molecular formula for a molecule. Draw the complete structure in the box to the right below. All the atoms in each molecule are closed shell (octets) and uncharged. Lack of IR data does not mean that a specific functional group is not present in the molecule.arrow_forwardQUESTION #1 PROPOSE ANSWER FOR THE FOLLOWING SPECTRA ? AND JUSTIFY YOUR ANSWERarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY