
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
draw products. all one question
![HCI
ΘΟΙ
Q
[1]
Select to Draw
Q
H₂C
Ⓒ
HCI
Q
Select to Draw](https://content.bartleby.com/qna-images/question/783921e3-4364-4a5c-bb85-82140d95faf9/d62e05d4-1c6e-4ecc-926a-7c0fbddd7484/kgb36rl_thumbnail.jpeg)
Transcribed Image Text:HCI
ΘΟΙ
Q
[1]
Select to Draw
Q
H₂C
Ⓒ
HCI
Q
Select to Draw
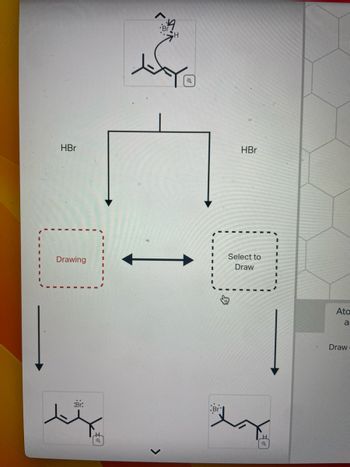
Transcribed Image Text:HBr
Drawing
ہتر
:Br.
H
Q
HBr
Select to
Draw
.Br
M
H
Q
I
I
I
I
I
I
I
Ata
a
Draw
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H Grubbs Catalystarrow_forward2. For the acid/base reaction below: a. Draw electron-pushing arrows to show how the reactants are converted to the products. b. Circle the side of the equilibrium that is favored. mot c. Draw a reaction coordinate diagram for the acid/base reaction. E reaction coordinatearrow_forwardI thought it was C&D because they needed more energy. Can someone explain pleasearrow_forward
- Draw curved arrows. Select Draw Templates More H₂C CH3 H₂C — : 0: CH₂ Erase heat H3CO + Ö-CH3arrow_forwardWondering how to do this one?arrow_forwardThe reaction diagram shown represents which type of reaction? Reaction Coordinate a. Two-step, slow, exergonic ab. Two-step, fast, endergonic o. One-step, fast, endergonic od. One-step, fast, exergonic e. One-step, slow, exergonic Energyarrow_forward
- for this question. Draw a structural formula for the missing product in the following reaction. 0. NaHCO3 H20 ? + CO2 + H20 + CH3C-OH • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Include cationic counter-ions, e.g., Na+ in your answer, but draw them in their own sketcher. C P. opy aste [F **** C - Visited CH4 ChemDoodle Retry Entire Group 5 more group attempts remaining Submit Answerarrow_forwardfor the reactants: ester and enolate ion. • Assume all esters to be ethyl esters. . Do not include counter-ions, e.g., Nat, It, in your answer. • Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple reactants using the + sign from the drop-down menu. ? O-Sn [Farrow_forwardDraw the arrows showing how electron move to get from reactant to product ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY