
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
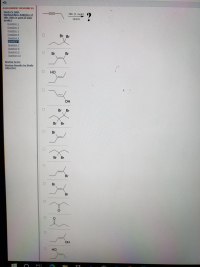
Transcribed Image Text:HBr (1 mole)
НООН
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hydrazine (N 2H 2) and hydrogen peroxide react to produce nitrogen and water. Howmany grams of hydrazine is needed to produce 87.0L of nitrogen?arrow_forwardLet suppose you have a large vat or container of I 2 (diatomic iodide), I tell you there is a total of 6 moles in some container. If there are 6 moles of the molecule I 2, how many individual moles of I exist in this same container? You could suggest none because it is all in the form of I 2 and that is not a bad answer, but thinking mathematically, from the information I gave you, how many moles of I (iodide) atoms exist in there?arrow_forwardHow many moles of Ag are in 63.5 grams of Ag₂O?arrow_forward
- A major component of honey is fructose (C6H12O6) with a molar mass of 180.16 g/mol. A tablespoon (15.0 mL) of honey is considered one serving. How many tablespoons must be eaten to consume 0.1900 moles of fructose? Assume honey is 100% fructose. The density of honey is 1.45 g/mL.arrow_forward3. For each acid, name the anion bonded to hydrogen, then name the acid. Anion Name Acid Name (а) НBr (b) НF (c) H;S (d) HNO; (e) HC10;arrow_forwardWhat is the total number of carbon atoms on the right-hand side of this chemical equation? CH,CO,C,H,(l)+H,O()−CH,CO,H(0)+C,H,OH()arrow_forward
- Be sure to answer all parts. Nitroglycerin (C;H;N3O9) is a powerful explosive. Its decomposition may be represented by 4C H;N3O9 6N2 + 12CO, +10H,0 02 This reaction generates a large amount of heat and gaseous products. It is the sudden formation of these gases, together with their rapid expansion, that produces the explosion. (a) What is the maximum amount of O, in grams that can be obtained from 8.00 x 10 g of nitroglycerin? (b) Calculate the percent vield in this reaction if the amount of O, generated is found to be 26.3 g. 0/0arrow_forwardComplete the reactions below with appropriate reaction conditions, make sure that your set of reaction conditions produce the indicated product as a major product. Please indicate clearly whether the reaction conditions occur in one or several distinct steps. Reaction Conditions Reactant Product a) Br b) OH c) HOarrow_forwardPlease help answer the subpart d, e, f and garrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY