
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
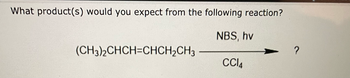
Transcribed Image Text:**Transcription for Educational Website**
---
**Organic Chemistry Reaction: Anticipating Products**
*Question:*
What product(s) would you expect from the following reaction?
*Chemical Reaction:*
(CH₃)₂CHCH=CHCH₂CH₃
- Reagents: NBS, hv, CCl₄
- Reaction Type: Allylic Bromination
*Analysis:*
The given reaction involves N-Bromosuccinimide (NBS), light (hv), and carbon tetrachloride (CCl₄), which are typical conditions for allylic bromination.
**Explanation:**
1. **Reagents Used:**
- **NBS (N-Bromosuccinimide):** Used for selective bromination at the allylic position.
- **hv (Light):** Initiates the formation of free radicals needed for the reaction.
- **CCl₄ (Carbon Tetrachloride):** Solvent that stabilizes the reaction intermediates.
2. **Mechanism Overview:**
- This reaction proceeds via a radical mechanism where light induces the homolytic cleavage of the N-Br bond in NBS to form bromine radicals.
- The bromine radical abstracts a hydrogen atom from the allylic position (the carbon next to a double bond), forming an allylic radical.
- The allylic radical then reacts with Br₂ (formed from reaction with NBS) to give the allylic brominated product.
3. **Expected Product:**
- Bromination occurs at the allylic position of the compound, leading to a substitution of a hydrogen atom with a bromine atom at this position.
---
This educational summary provides an understanding of allylic bromination and the expected outcome of the given reaction under specific conditions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are the final products. Show stereochemistry if needed.arrow_forwardWhat is the expected product of the following reaction? 1. Br2/H20 2. NaOH Br II II + enantiomer + enantiomer IV O II OIV Ovarrow_forwardWhat is/are the product(s) of this reaction? 1 Hg(OAci CH,OH 2 NABH, HO enantiomer OCH enantiomer OCH2 D.arrow_forward
- Predict the product(s) of the following reactions, including stereochemistry when necessary and identify the mechanism of each substitution reaction (SN1 vs SN2). Draw the reaction mechanism (reaction arrows) for any one of the reactions to show how the product is formed.arrow_forwardDraw a structural formula for the product of each SN2 reaction. Where configuration of the starting material is given, show the configuration of the product.arrow_forward7. f Which of the following is the product of the reaction of 1-hexyne with 1 mol of Br₂? (a) Abi (c) Br Br Br Br Br Br (e) Br Br Br Brarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY