
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
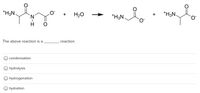
Transcribed Image Text:of
*H3N.
H20
*H3N.
+
+
*H3N.
The above reaction is a
reaction.
condensation
hydrolysis
O hydrogenation
hydration
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What reactant would transform the molecule from the top left to the top right. Given the same molecule, what would the given reactants transform it into below?arrow_forwardIn comparing a reaction run under reflux versus distillation, distillation will remove the product, as it's formed. Are we using thermodynamics or kinetics to drive the reaction? Only kinetics because the raised temperature makes the reaction occur faster Only thermodynamics because we're removing products, Le Chatlier would describe this as a way to favor products Both kinetics and thermodynamics are favored Neither kinetics or thermodynamics are favoredarrow_forwardAn increase in water temperature is observed when 1.97 g of NaOH is added. The enthalpy change for this reaction is -2.19 kJ. Calculate the AH°scln for NaOH. The AH°scln for NAOH is -44.5 kJ/mol. The AH° soln for NaOH is -26.3 kJ/mol. O The AH°scln for NaOH is -72.4 kJ/mol.arrow_forward
- 128 CHAPTER 5 3. A chart of vapor pressure information at different temperatures for various liquids is given below. Vapor Pressure (torr) Temperature Water Ethyl Alcohol Carbon Tetrachloride O'H 4. 24 (C) HOʻHƆ 12 34 0. 25 116 09 224 95 316 75 294 675 731 760 1723 1486 The questions below refer to the following experiment: three flasks, each containing one of the three liquids listed in the table are hooked to a vacuum pump. The pressure in each flask is reduced to 50 torr and the temperature of each flask is maintained at 25°C. blano (b) (a) Under these conditions, which liquid(s) would you observe boiling? Explain. (b) Explain why, if both liquids are maintained at 25°C, the vapor pressure of CCI, is higher than the vapor pressure of C,H,OH. Refer to both molecules in your discussion. CH H'C CH enstud-S-otoahib-CS-uot OBO 2ADI ud,8gada al sanm alom omss odi ovard asonatadue dhol () palom alimis bas Dwal s ad mot ab at a tmioq gaiilam Dwal inlqx ToonanoROT atidio ym li mol diW )…arrow_forwardTo prepare 0.20 M NaOH (40.0 g/mol) you will need to dilute 34 g of NaOH to ____ mL. a.2.6 mL b.4.2 mL c.4200 mL d.170 mL e.590 mLarrow_forwardQuestion 4 of 7 Submit Draw the major product of this reaction. Ignore inorganic byproducts. CI excess CHзNH2 Select to Drawarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY