
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Explain the difference between the two reactions-why are the products different?
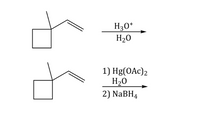
Transcribed Image Text:H30*
H20
1) Hg(OAc)2
H20
2) NaBH4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For a chemical reaction, which conditions will result to the formation of the product? Choose from the choices below: Low kinetic energy and more collision among molecules. High kinetic energy and more collision among molecules. Low kinetic energy and proper orientation among molecules. High kinetic energy and proper orientation among molecules.arrow_forwardUsing your knowledge of temperature and reaction rate, explain why food spoils at a slower rate when refrigerated.arrow_forward⦁ 4. Make your own energy diagram for an exothermic reaction that does not include a catalyst and one with a catalyst (Be sure each line is a different color or labeled). Label the axis of the diagram. Then, state what a catalyst is and how a catalyst affects the reaction rate.arrow_forward
- How does Le Chatelier’s principle impact the example of equilibrium in daily life shown in the image?arrow_forwardWhich one of the following reactions belongs to an exothermic reaction? A. The ionization of potassium atom B. The breaking of a C-C bond C. The condensation of water vapor D. The sublimation of sodium metalarrow_forwardBalance the following reaction and write the reaction rate. __CH3NH2(g) + __O2 --> __CO2(g) + __H2O(g) + __N2(g)arrow_forward
- Magnesium (Mg) is placed in a test tube containing hydrochloric acid (HCI). Will a reaction take place? * Yes O Noarrow_forwardIf methylisonitrile is placed in a sealed quartz tube and heated in a tube furnace, it isomerizes to acetonitrile according to the reaction below: CH3NC (g) → CH3CN (g) The sealed tube initially contains 0.200 mol of methylisonitrile and zero moles of acetonitrile. After about 25 minutes of heating, there is 0.108 mol of methylisonitrile remaining in the tube. Please calculate the average rate of decomposition of methyisonitrile during this 25 min. period. Question 3 options: A) 3.7×10-3 mol/min B) 0.092 mol/min C) 4.3×10-3 mol/min D) 2.3 mol/min E) 0.54 mol/minarrow_forwardThursday, December 14, 2023 4:49 PM OH CH3 Br CH3 Complete the following reaction.arrow_forward
- What type of reaction is this? In this diagram, what is the potential energy of C? In this diagram, what is the potential energy of D? In this diagram, what is the Eд without an enzyme? In this diagram, what is the Eд with an enzyme? In this diagram, what is the AG? 250 200 PE EA1 150 (kJ) D 100 "E с 50 Reaction pathwayarrow_forward1. Reactions that occur in the gaseous state are affected by changing the volume of the container. Which choice best describes what will happen to the reaction if the volume of the container is decreased? The reaction will shift right. The reaction will shift left. The reaction will not change. The reaction will speed up.arrow_forwardThe sum of all anabolic and catabolic processes in a cell or organism 1. energy Measure of the stability of 2. metabolism a chemical bond 3. bond energy Energy that can do useful work 4. respiration Randomness or disorder in a collection of objects or 5. equilibrium energy 6. potential energy Conditions in which bonds 7. activation energy are breaking within reactants and forming between products 8. reactionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY