
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
synthesis of banana oil
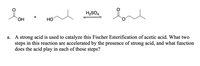
Transcribed Image Text:H2SO4
+
HO,
HO
a. A strong acid is used to catalyze this Fischer Esterification of acetic acid. What two
steps in this reaction are accelerated by the presence of strong acid, and what function
does the acid play in each of these steps?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following chemical reaction is used to synthesize a flavouring agent that has an aroma similar to bananas. H₂SO4(aq) CH3COOH(1) + CH3(CH₂)₂OH(1) I || Identify the type of reaction that is represented by this synthesis. Select one: CH₂COO(CH₂)₂CH₂(1) + H₂O(1) IV O addition O hydrogenation O substitution O esterification O eliminationarrow_forward5. Which of the following equations shows that isoquinoline, C9H;N, behaves as a Bronsted-Lowry base in water? a) CóH;N(aq) + H2O(1) = b) CH;N(aq) + H2O(1) c) C9H;N(aq) + OH¯(aq) = d) CoH;N(aq) + H;O*(aq) e) CoH;NH*(aq) + H2O(!) C9H;NH*(aq) + OH (aq) = C9H&N°(aq) + H;O*(aq) C9HN¯(aq) + H;O(!) = C9H;NH*(aq) + H2O(1) = C9H;N(aq) + H;O*(aq)arrow_forwardFor the following reaction scheme, identify by drawing the reagents a, c, and d and the intermediate b that are formed in the synthesis of 2-phenylethanoic acid.arrow_forward
- potassium dichromate (Cr2O7^2-) oxidation of ethanol to acetic acid is the basis for the original breath-alcohol screening test used by law enforcement agencies to determine a person's blood alcohol content (BAC). the test is based on the difference in colour between the dichromate ion( redish orange) in the reagent and the chromium(iii) ion (green) in the product. deduce the reaction that is involved in this test and give the IUPAC name of the other product formed.arrow_forwardOne of the steps in fat metabolism is the hydration of crotonate to yield 3-hydroxybutyrate. This reaction occurs by addition of —OH to the Si face at C3, followed by protonation at C2, also from the Si face. Draw the product of the reaction, showing the stereochemistry of each step.arrow_forwardSodium borohydride (NABH4) is said to be a chemoselective reducing agent. Which of the following best describes what this term means? O 1 A reaction that operates exclusively on one functional group in the presence of other functional groups. A reaction that operates on one functional group at a time and is shut down 2) when other functional groups are present. O 3) A reaction that does not operate at all in the presence of a functional group. OA reaction that operates on multiple functional groups in the presence of 4) multiple functional groups.arrow_forward
- Given the following compounds and boiling points: Determine the IMFs Order the compounds from weakest to strongest IMFs, and Pentane (C5H12) 36°C Methane (CH4) -164°C Sodium n-butoxide (C4H9NaO) +260°C Hydrogen dioxide (H2O) 100°Carrow_forward7A Write the possible products of the following reactions, the mechanism by which they were formed. mentioning Br CH3OH CH3ONaarrow_forwardFind the AHrn for the following reaction: 2N29) + 502(9) → 2N,O5(9) given the following reactions and subsequent AH° values 2H2(9) + O2(9) → 2H,Ou) N½O5(9) + H2Ou → 2HNO3(1) AH° = -541.6 kJ AH° = -54.6 kJ %D N2(9) + 302(9) + H2(9) → 2HNO3(1) AH° -331.1 kJarrow_forward
- Please name the following compounds.arrow_forwardGive the major organic product(s) for each of the following reactions. Write NR if a reaction will not occurarrow_forwardThere is a tick and mosquito repellent DEET (diethyltoluamide) that is prepared by a reaction of diethylamine with m-methylbenzomic acid (m-toluic acid) H+ and heat. What's the structure of DEET?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax