
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Use the activity series to predict if the following reactions will occur. If the reaction will occur, predict the products (write the correct formulas) and balance the equation. If the reaction is not possible, all you have to do is write NR (no reaction) after the arrow
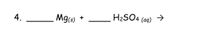
Transcribed Image Text:4.
Mgs)
H2SO4 (aq) →
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction, do the following: 1. Write the correct formulas for the products, assuming a metathesis/exchange/double replacement/double displacement reaction is occurring 2. Using a solubility table, predict whether the reaction would produce a precipitate and fill in the phase subscripts for each product 3. Balance the equation, giving the correct balanced molecular equation 4. Write the total ionic equation 5. Write the net ionic equation FeCl3 (aq) + K2S (aq) →arrow_forwardConsider a double replacement reaction between Zinc (ll) chloride and sodium hydroxide: ZnCl2+NaOH ==> Complete the chemical equation by: 1. Rewriting it to include electrically-neutral ionic product formulas for each formula. To clarify instead of just writing the product formulas rewrite the entire equation. Concerning the "electrically neutral" part, you are reminded that if for example, Na + Cl2 ==> were your reactants, you would not predict NaCl2 as a chemical product because that is not an electrically neutral compound formula. The Na would have a +1 charge and, the Cl would have a -1 charge, 2 (-1) charges would give an overall -2 charge which would not produce an overall 0 charge. 2. Balance it. 3. Add subscripts (aq, I, s, or g) to show how the reactants combine and which product will precipitate to be collected as a solid. 4. Provide subscripts for both reactants and both products.arrow_forwardPlease write the answer clearly with the state of matter following each compound.arrow_forward
- Which of the following correctly classify the following reaction? (There could be more than one answer) Zn(s) + 2HCl(aq) --> H2(g) + ZnCl2(aq) Acid/Base reaction Combustion reaction single displacement reaction double displacement reaction gas evolving reactions precipitation reactionarrow_forwardplease help me fill out this chartarrow_forwardBalance the following reaction in basic solution then enter the coefficients of the balanced reaction into the blank spaces. Remember to include "understood" coefficients of 1! 9. Type your answer here Ag20(s) + Type your answer here Al(s) + Type your answer here OH (aq) + H20(1) → Type your answer here Type your answer here Ag(s) + Type your answer here H2A!O3 (aq)arrow_forward
- Write the (i) balanced conventional equation (CE), (ii) total ionic equation (TIE), and (iii) net ionic equation (NIE) for each of the reactions below. Include all state symbols. Assume that, if there is a reaction, it will go to completion. If there is no reaction, write "No Reaction" for the NIE but still complete the CE and TIE. a. Aqueous solutions of potassium sulfate and calcium iodidearrow_forwardTech Please don't provide the handwriting solutionarrow_forwardFor each of the following sets of reactants, write the balanced equation for the double displacement reaction that occurs. If you determine that a reaction will not occur, write "NR", and provide a brief explanation. Aqueous zinc chloride + aqueous sodium chromate Aqueous lithium hydroxide + phosphoric acidarrow_forward
- Ammonium Chloride+ sodium hydroxide --> Molecular equation, Total ionic equation, net ionic equation, and type of reaction.arrow_forwardWrite the word equation below and determine the type of reaction (synthesis/combination,decomposition,single-displacement,double-displacement or combustion. 2H20=2H2+02arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY