
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Just need help with the last two 3,4
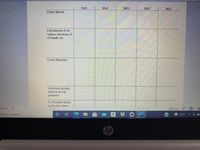
Transcribed Image Text:H20
NO,+
PO,3-
NO
Crude Sketch
Calculations (# of
valence electrons, #
of bonds, etc.
Lewis Structure
# electron groups,
electron group
geometry
# of bonded atoms,
molecular shane
28 words
OFocus
e here to search
a *
85°F A O
hp

Transcribed Image Text:FHeading 4
A Select
Font
Paragraph
Styles
Editing
# electron groups,
electron group
geometry
# of bonded atoms,
molecular shape
Resonance
structures (if any)
Polar or nonpolar
7.
OFocus
2428 words
o 耳
85°F A a
a
ype here to search
hp
12
17
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 21. How many milliliters of 18.2 MH₂SO4 are needed to prepare 600.0 mL of 0.10 MH₂SO4? a. 0.30 mL b. 109 mL c. 3.3 mL d. 1.6 mL e. 4.3 mLarrow_forwardMatch the following descriptions in column A to the choices listed in column B: A 1. Stoichiometry 2. Spikes 3. Ferromagnetism 4. Colloid 5. Hole 6. Nanoparticle 7. Empirical formula 8. Unit cell 9. Surfactant 10. Ferrofluid 11. Magnetite 12. Van der Waals forces B a. Weak forces of attraction between molecules b. Regions where unpaired electrons strongly interact with one another & align, even in the absence of a magnetic field c. A pattern of uplifted suspended particles that arise from placing a magnet near ferrofluid d. A chemical term that deals with relative amounts of substances involved in chemical reactions e. A dispersion of particles from ~ 1 nm to 1000nm f. A phenomenon in which the internal magnetic moments of unpaired electrons within the domain of the solid are aligned and act cooperatively g. An empty site in a crystalline solid h. A very small particle on a scale of nanometers (10⁹m) i. The name for Fe3O4 j. Information that gives the simplest ratio between atoms of…arrow_forwardEvaluate the product. (7.61×10^9)(4.46×10^3)arrow_forward
- A 0.200 M K2SO3 solution is produced by A) diluting 250.0 mL of a 1.00M K2SO3 solution to 1.00 L B) diluting 100.0 mL of a 2.50 M K2SO3 solution to 250.0 mL C) diluting 20.0 mL of a 5.00 M K2SO3 solution to 500.0 mL D) diluting 1.00 mL of a 250M K2SO3 solution to 1.00 L O B C OD O Aarrow_forwardA doctor has ordered for a week 0.35 grams of Carvedilol for a patient with high blood pressure. If your stock on hand consists of 25 mg tablets, how many tablets will you need for 1 day's treatment for the patient? QUESTION 13 O a. 2 O b. 14 O C. 7 O d. 20 O e. 1 QUESTION 14arrow_forwardAll changes saved 6. You have a very concentrated solution (12 M) of potassium chloride (KCI). You need it to be at the lowest concentration possible for the experiment you are about to conduct. The problem is you forgot to order lab supplies, so you only have 2 L of distilled water left. What would be the final concentration if you added the two 2 L of distilled water to the 0.5 L of 12 M KCI? O 3.0 M 48 M 24 M O 2.4 M PREVIOUS 6 of 20 NEXTarrow_forward
- Part IV. Henry's Law More Carbon dioxide gas will dissolve (or stay dissolved) in water when pressure is (high or low?) .Dissolved Carbon dioxide leaves water when the pressure is (high or low?)arrow_forwardA 1:20 dilution is done with a 0.2500 M Na,SO, solution. What is the concentration of the diluted solution ? A. 0.5000 M B. 0.0500 M C. 0.1250 M D. 0.01250 Marrow_forwarda) Determine the concentration of acetic acid in the 25.00 mL aliquot. b) Determine the concentration of acetic acid in the 10.0mL of vinegar. concentration NaOH = 0.101M Trial 3 Initial NaOH level 35.1 mL Final NaOH level 52.5mL The volume of NaOH used to reach the endpoint 17.4 mLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY