
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
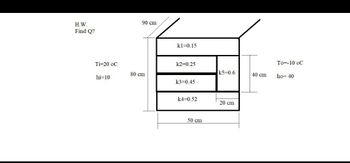
Transcribed Image Text:H.W.
Find Q?
Ti-20 oC
hi=10
90 cm
80 cm
kl 0.15
k2=0.25
k3=0.45
k4=0.52
50 cm
k5=0.6
20 cm
40 cm
To -10 oC
ho= 40
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- About how many liters (@STP) does 1269 grams of I2 occupy? 2240 liters 224 liters 1120 liters 112 liters None of the abovearrow_forwardWhat is the correct IUPAC name of the following compound? No capitalization, no italization, no spaces.arrow_forward80 • 53 °C A 63 °C -75,6 "C x -82.7 "C A -100 °C Triple Point --- Predicted •59.4 °C - 70 "C o 80 °C O 90 "C - -120 "C --Critical Locus 70 60 * ** * * 40 30 20 -75.6 C 41 10 110°C 0.76 0.78 0.8 0.82 0.84 0.86 0.88 0.9 0.92 0.94 0.96 0.98 1 Mole fraction of CH, in vapor phase At 20 bar and -90 Celsius At 40 bar -63 Celsius At 10 bar and -90 Celsius At 50 bar and -53 Celsius CH4 in vapor 0.97 .A CH4 in vapor 0.85 .B Pressure (bar)arrow_forward
- Using the data below, construct a calibration curve. Include the error bars and the equation of the line and the linearity (R2) of the curve. Calcium concentration (ppm) (X-data) Absorbance (Y-data) Run 1 Absorbance (Y-data) Run 2 Absorbance (Y-data) Run 3 0.3 1109 1069 1155 1.2 1225 1168 1233 2.1 1472 1319 1389 4.2 1497 1523 1472 8.0 1833 1898 1849 16 2066 2012 2051arrow_forwardCan a small sample of one substance have the same mass as a large sample of a different substance? Explain/give simple examplesarrow_forwarduse resonance structure to explain which structure is more stablearrow_forward
- How many Oxygen atoms would be produced from 18.75g Al(NO3)3? digit_______ (format of answer: 1.23x10^23) unit ________ Blank 1: Blank 2:arrow_forwardAnswer the following questions and show your complete solution. This means the studentt must include drawings or schematics if necessary. 1. Calculate the volume of a BCC unit cell in terms of the atomic radius R. 2. The atomic packing factor (APF) for a BCC unit cell is 0.68. Show how this value was obtained/calculated.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The