
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Lactic acid is a natural compound found in sour milk.
(a) How many π bond occur in lactic acid? How many u bonds?
(b) What is the hybridization of atoms 1, 2, and 3?
(c) Which CO bond is the shortest in the molecule ? Which CO bond is the strongest?
(d) What are the approximate value of the bond angles A, B and C?
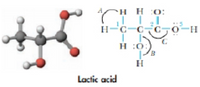
Transcribed Image Text:H.
H :0:
H-C
-H-
H :0
Lactic acid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H2CO molecules (a) use orbital hybridization theory to determine the molecular shape of h2co. (b) what bonds are formed between the c and o atoms in formaldehyde molecules?arrow_forwardDraw the Lewis structure of CH₃CCH and then choose the appropriate set of hybridization states for the three central atoms. Your answer choice is independent of the orientation of your drawn structure.arrow_forward1. For each molecular geometry, give the number of total electron pairs, the number of bonding pairs and the number of lone pairs on the central atom. (a) (b) (c) 2. For the following species provide the Lewis structure and make a 3D sketch showing estimated bond angles. Determine the polarity of each molecular and draw the dipole. a) SF4 b) KrF4 3. Determine the 3D structure of the molecule of formula C2H4O (H3CCHO). Indicate the geometry around each "central" atom and estimate bond angles. Representing Molecular Geometries on Paper X-A-X XX Linear Trigonal planar Bent Tetrahedral Trigonal pyramidal Trigonal bipyramidal Seesaw Octahedral Square planararrow_forward
- Draw the Lewis structure of methanethiol (CH₂SH) and then choose the appropriate pair of molecular geometries of the two central atoms. Your answer choice is independent of the orientation of your drawn structure.arrow_forwardI have a question regarding hybrid vs atomic orbitals: 1) What would be the hybridizations of C and O in the molecule H2CO? It asks to draw an electron orbital diagram (with arrows) of all the electrons in C before and after hybridization. 2) Draw a diagram of the H2CO molecule showing the overlap of orbitals between the C and O atoms that make up the sigma (σ) and pi (π) bonds. Which orbitals make up the σ bond? Which ones form the π bond?arrow_forward(a) Using the molecular orbital model, write the valence electron configuration for CN and use it to calculate the bond order. Is the species paramagnetic? Bond order = Paramagnetic? (b) Using the molecular orbital model, write the valence electron configuration for CO and use it to calculate the bond order. Is the species paramagnetic? Bond order = Paramagnetic?arrow_forward
- (a) Describe the hybridization of the central atom of a molecule with a see-saw shape. (b) Describe the hybridization of the central atom of a molecule with a trigonal planar shape. (c) Describe the hybridization of the central atom of a molecule with a trigonal bipyramidal shape.arrow_forward2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY