
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The unmistakable odor of a freshly cut cucumber is largely due to cucumber
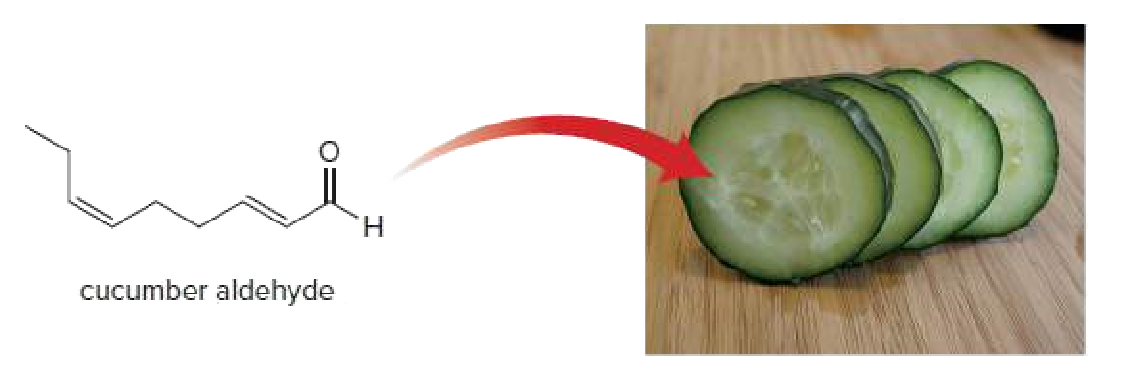
Transcribed Image Text:H.
cucumber aldehyde
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 7. Please identify the hybridization of carbon in the following compounds. (а) CH4 ER}=CH2 HCECAarrow_forward(a) Draw Lewis structures for ethane 1C2H62, ethylene1C2H42, and acetylene 1C2H22. (b) What is the hybridizationof the carbon atoms in each molecule? (c) Predict whichmolecules, if any, are planar. (d) How many s and p bondsare there in each molecule?arrow_forwardTwo isomers share the molecular formula C3H4. In Structure A two ofthe carbon atoms are sp hybridized and one carbon atom is sp3 hybridized. In Structure B, two of the carbon atoms are sp2 hybridized and one carbon atom is sphybridized. b)Draw “3D”-looking structures on paper, make a sketch of each of the isomers. (This should be a sketch of themolecules, not diagrams of overlapping orbitals!) Your drawing should show thecorrect molecular geometry around each carbon atom. [HINT: NEITHER of thesemolecules is planar!!! To draw them correctly, you will have to consider why!]arrow_forward
- 17) What are the "theoretical" values for the C−C≡C bond angles?arrow_forward13. (a) For the following condensed formulas draw the Lewis structures. Include lone pairs in your drawing, 03 (ozone) CH3COOH (b) Write in any non-zero formal charge (c) Give the molecular geometry around the second carbon in CH3COOH (d) What is the hybridization of the second carbon in CH3COOH (e) Give the molecular geometry around the middle O in O3. (f) What is the hybridization of the middle O in O3 14. Draw the line structure of the following compounds? a) (CH3)3CCH₂CH₂NHCH3 15. a. Draw two constitutional isomers with the formula C₂HgBrCl Isomer 1 16. For the molecule below, b) CH3CHO b. Describe ALL the intermolecular forces isomer 1 can participate in OH Isomer 2 3 (a) Circle all functional groups present. (b) Label two of the functional groups next to where you circled themarrow_forward2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY