
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Draw the perspective structure . Lone pairs must be written
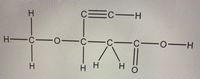
Transcribed Image Text:H.
Ec
C-H
H -C
C
H-
H.
Expert Solution
arrow_forward
Step 1
- Lone pair:- These are the non-bonding electrons, which are present on atoms.
- Lone pair makes an atom an electron rich molecule.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw resonance structures for the following compound: Add curved arrow(s) to show resonance using one of the five patterns, and modify the second structure given to draw the new resonance structure. Include relevant formal charges in your structure. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. H₂C CH3 N. H3C CH3 Edit Drawingarrow_forwardAdd the unshared lone electron pairs to the following structure.arrow_forwardDraw the molecular shape of SeCl2. Make sure to use solid wedge and dash bonds if needed.arrow_forward
- Provided is the first and last resonance. Draw the missing resonance forms, then propose the corresonding resonance hybrids of each molecule. Show the movement of electrons using appropriate arrows.arrow_forwardDraw the line structure of eugenol and state the molecular geometry of every carbon and oxygen. This is not an exhaustive list but choices include tetrahedral, bent, linear, trigonal pyramidal, and trigonal planararrow_forwardwhy does hydrosulfuric acid contain two hydrogens?arrow_forward
- a) What would the formula (CxHy) of the compound cyclobutyne be? b) What is the hybridization of the carbon atoms in the triple bond? What bond angle is this normally associated with? c) Cyclobutyne is not a stable compound and has never been observed. Suggest a reason why.arrow_forward6arrow_forwardPlease help me out, indicate bond angles at the highlighted positions.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY