
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
State if they are enantiomers, diastereomers, consitutional, or identical.
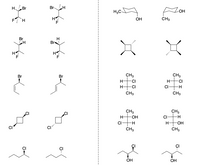
Transcribed Image Text:H.
Br
Br H
H3C-
OH
FH
OH
CH3
Br
Br
F
Br
Br
CH3
CH3
H-
CI
H-
CI
H-
CI
H-
CH3
CH3
CH3
CH3
CI
H-
OH
CI-
-H-
CI
H-
H-
OH
CH3
CH3
OH
OH
Expert Solution
arrow_forward
Step 1
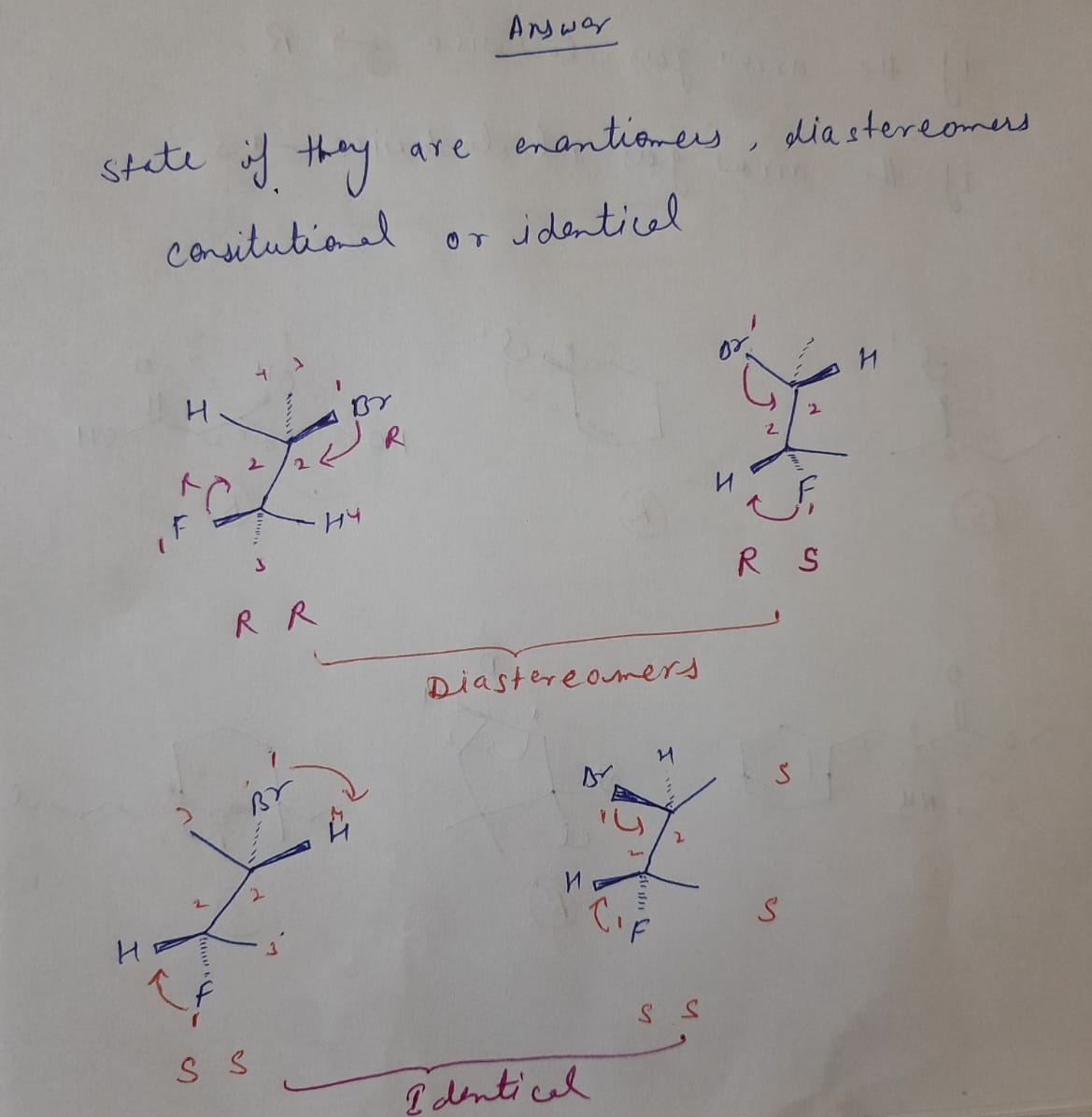
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Determine the relationship between the two drawings: diastereomers enantiomers same compound O constitutional isomersarrow_forwardC/ What is the relationship between these two structures? CI same enantiomers constitutional isomers diastereomersarrow_forwardEnantiomers are a pair of molecules that are non-superimposable mirror images. Identify the two molecules that are a pair of enantiomers. Br Cobr CI Compound 1 'Br Compound 3 CI Br Compound 2 CI 'Br Compound 4 A) Compounds 1 and 2 B) Compounds 1 and 3 C) Compounds 2 and 3 D) Compounds 1 and 4 E) Compounds 3 and 4arrow_forward
- The relationship between the following molecules is best described as : NH2 H,N° Differents Enantiomers diastereomers O identicalsarrow_forwardWhich of the following correctly describes the relationship of the molecules shown to Compound A? HO,C CI Compound B coM HO,C Isomer A CI Compound C CI Compound D B = meso (identical) C = diastereomer D= diastereomer B= diastereomer C = meso (identical) D= diastereomer B = meso (identical) C = enantiomer D= diastereomer B = diastereomer C = diastereomer D= enantiomerarrow_forwardWhich of the following is the definition of chirality? A) The existence of a molecule with a mirror image B) The existence of a molecule that is superimposable on its mirror image C) The existence of a molecule that is not superimposable on its mirror imagearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY