
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give detailed Solution with explanation needed..don't give Handwritten answer....
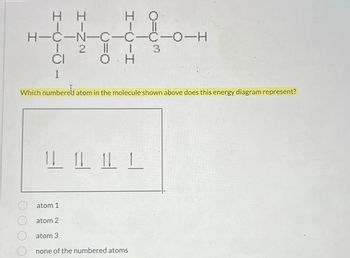
Transcribed Image Text:H H
о
H-0-0-0-0-N-O-H
2
CI
0
3
Which numbered atom in the molecule shown above does this energy diagram represent?
111
atom 1
atom 2
atom 3
none of the numbered atoms
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What examples sampling tests, information, data in regards to salt content be used for class mean in both regular and unsalted tomato sauce please?arrow_forwardraw Hill C C Chegg - Get 2 MOTION EYE OWLv2 | Assig Submit Answer Content gagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator assignment-take OWLv2 | Orx Retry Entire Group [Review Topics] [References] Use the References to access important values if needed for this question. CengageNOW An aqueous solution is made by dissolving 17.2 grams of iron(II) chloride in 431 grams of water. The molality of iron(II) chloride in the solution is m. e 4 more group attempts remaining New Tabl D →1 D 2. Other Bocarrow_forwardHelp me answer thisarrow_forward
- What is the number of equivalents of Fe3+ in 2 moles of Fe³+? Osoft olga gamboa Bobby Offer ge retirement a... of Employ... Scaux Cave chemistry Bobby Offer Painting and reaction of Employ... 83°F Sunny bbbbbbbbbbbb....arrow_forwardA beer sample is analyzed in the laboratory to determine the international bitterness units (IBUs). The analysisis performed in triplicate with the following results: 22.5, 25.4, and 21.7. Within what range are you 95%confident the true value lies?arrow_forwardMatch the following terms to their definitions: Aqueous solution, cation, centrifuge, decant, flame test, precipitate, qualitative analysis, supernate the systematic separation and identification of the chemical components in an unknown sample is known as ...? Group of answer choices Centrifuge Decant Supernate Flame Test Aqueous Solution Precipitate Qualitative Analysis Cationarrow_forward
- Letter B.... please show step by step solutionarrow_forwardSelect one for each boxarrow_forwardAM McGraw Hill C C Chegg- Get 2 MOTION EYE OWLv2 | Assig Submit Answer //east.cengagenow.com/ilrn/takeAssignment/takeCovalentActivity.do?locator=assignment-take Content OWLv2 | Onx mm Hg [Review Topics] [References] Use the References to access important values if needed for this question. Retry Entire Group CengageNOW The vapor pressure of benzene is 73.03 mm Hg at 25 °C. A nonvolatile, nonelectrolyte that dissolves in benzene is testosterone. D Calculate the vapor pressure of the solution at 25 °C when 9.655 grams of testosterone, C19H2802 (288.4 g/mol), are dissolved in 212.0 grams of benzene. benzene = C6H6 = 78.12 g/mol. VP(solution) 4 more group attempts remaining New Tab Otharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY