
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please be clear in your writing
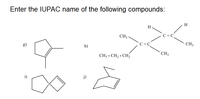
Transcribed Image Text:Enter the IUPAC name of the following compounds:
H
H
CH3-
C = C
C = C
CH3
g)
h)
CH3
CH3 - CH2 - CH2
i)
j)
Expert Solution
arrow_forward
Step 1
For naming in IUPAC follows following rules:
- The longest chain rule dictates that the parent hydrocarbon be found and then named. The parent chain of the molecule in question is usually the longest carbon atom chain, whether it is straight or a chain of another shape.
- The locants with fewer numbers: the carbon atom in the parent hydrocarbon must be numbered using natural numbers, starting at the end with the carbon atom that bears the substituents receiving the lowest number.
- There are several instances with the same substituent: prefixes such as di, tri and others are used to indicate the total number of the same substituent in the supplied chemical compounds.
- Naming of distinct substituents: In organic compounds with several substituents, the appropriate substituents are listed in the IUPAC nomenclature in alphabetical order.
- Various substituents at the same location are given different names: the substituents are named in ascending alphabetical order when two different substituents are present at the same location of the organic compound.
- Complex substituents in organic compounds with branched structures must be referred to as substituted aklyl groups, with the carbon linked to the substituent group being numbered as one. In the IUPAC nomenclature of related compounds, these branching and related compounds must be written in brackets.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- B File Paste E + Home Cut E Copy 4 Page 1 of 1 Clipboard Format Painter Insert 0 words S Draw Design Layout References Calibri (Body) BIU U abe 11 À A Aa A A abe X₂ X² Font Type here to search P English (Philippines) Accessibility: Investigate Mailings 10 E•E•S• Review View Help HE e W Document1 - Word €¶ Paragraph 1-32. A bottle of concentrated aqueous sulfuric acid, labeled 98.0 wt% H₂SO4, has a concentration of 18.0 M. (a) How many milliliters of reagent should be diluted to 1.000 L to give 1.00 M H₂SO4? (b) Calculate the density of 98.0 wt% H₂SO4- S ■ Tell me what you want to do AaBbCcDc AaBbCcDc AaBbC AaBbCct AaB AaBbcct AaBbCcD Normal 1 No Spac... Heading 1 Subtitle Subtle Em... Heading 2 Title Styles Sign in 1:31 (Ctrl) - ENG S IX Share Find ab Replace Select Editing 8:09 pm 18/08/2022 ▸► + 228%arrow_forward[References] 1 pt b. Balanced formula equation: 1 pt HCHO2(aq) + KOH(aq) 1 pt 1 pt Complete ionic equation: 1 pt 1 pt 1pt 1 pt Net ionic equation: 1 pt 1 pt 1 pt c Balanced formula eqmation: 92) e element +. +.arrow_forwardShow ALL your steps and details in your calculations, by using GRASPS: Given, Required, Analysis, Solution, and Paraphrase. Answer in complete sentences and therefore statements. Show All of your work, in full solutionsarrow_forward
- O Course Home b Chemistry Question | bartleby G indivuials with austium good to -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001 Q * E Apps O Maps E Connect - To Do As... O OCCC Moodle P chem work b help Gmail YouTube Balance Chemical E. II Review | Constants | Periodic Table A carbon atom is chiral if it is bonded to four different groups. For example, CHCIBII is chiral, but CCl,BrI is achiral because some of the bonded groups are the same. If a chiral carbon atom is present, then that molecule has a non-superimposable mirror image called an enantiomer. (Figure 1) Part A Identify all the chiral atoms in the below structure by right-clicking* a chiral atom to bring up a menu that includes "Atom Properties." Click on Atom Properties then click the checked box next to the Map field to clear the checkmark. Then enter "1" in the Map *Mac users: Use an equivalent for right- field box to label that chiral carbon atom. clicking.…arrow_forwardPlease don't provide handwritten solution .....arrow_forwardA. Consider the endothermic reaction at 25oC: FeSCN+2(aq) --><-- Fe+3(aq) + SCN-(aq) What direction will the reaction shift in response to the following stresses? (Use arrows or write "right" , "left" , or "no shift"). a. More SCN- is added. b. Solid sodium hydroxide is added. (iron (III) hydroxide is insoluble). c. The reaction container is set into ice water to cool. d. The pressure of the air around the reaction is doubled. e. Solid iron (III) chloride (FeCl3) is added. B. At 25oC, Kc for thr above reaction is 9.1 x 10-6. Determine the equilibrium concentration of Fe+3 in the solution if 0.50 mol of FeSCN+2 is dissolved to make 2.50 liters of solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY