
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
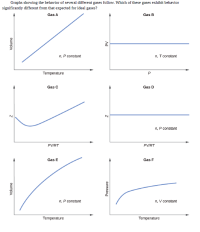
Transcribed Image Text:Graphs showing the behavior of several different gases follow. Which of these gases exhibit behavior
significantly different from that expected for ideal gases?
Gas A
Gas B
n, P constant
n, T constant
Temperature
Gas C
Gas D
n, P constant
PVIRT
PVIRT
Gas E
Gas F
n, P constant
n, V constant
Temperature
Temperature
Volume
Volume
Pressure
Ad
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Similar questions
- a. Given the following set of values for a gas, calculate the unknown quantity: P = 743.3 mm Hg V = ? L n = = 0.1021 mol T = 23.3 °C V = L b. Given the following set of values for a gas, calculate the unknown quantity: P = ? atm V = 28.4 mL n = 0.007052 mol T = 20.5 °C P = c. Given the following set of values for a gas, calculate the unknown quantity: P= 1.079 atm T = atm V = 44.7 mL n = 0.003411 mol T = ? K Karrow_forward1. e An 8.00 L sample of an ideal gas at 25° C undergoes a change under an external pressure of 303.9 KPa while during the process. a) Is the final temperature of the gas sample equal to 25° C, lower than 25° C, or higher than 25°C? Explain your answer. b) Calculate the final volume of the gas sample.arrow_forwardConsider the following samples of gas: sample composition pressure temperature A 2.0mol Xe (g) 1.7atm 228.°C B 2.0mol Xe (g) 1.4atm 258.°C C 2.0mol Xe (g) 1.6atm 280.°C sample composition pressure temperature D 1.5mol He (g) 1.5atm 41.°C E 1.5mol Ar (g) 2.4atm 41.°C F 1.5mol Ne (g) 1.2atm 41.°C Draw the set of graphs below that show the distributions of the speed of the atoms in each sample.arrow_forward
- Jj.118.arrow_forwardA gas occupies 3.00 L at 55.0°C. What is the temperature of the gas when its volume expands to 6.00 L (assuming the P is constant)? 110 °C 656 °C 343 °C 27.5°C 383 °Carrow_forward19. 1.18 g of Ar(g) occupies 2.00 L. container at the temperature of 25 °C. Calculate the pressure in mmHg. molar mass of Ar = 39.95 g/mole O 22.1 mmHg O 275 mmHg O 118 mmHg O 0.0156 mmHgarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY