
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
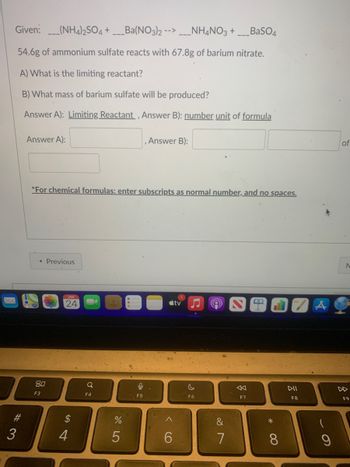
Transcribed Image Text:Given:(NH4)2SO4 +___Ba(NO3)2 -- ____NH4NO3 +___BaSO4
54.6g of ammonium sulfate reacts with 67.8g of barium nitrate.
A) What is the limiting reactant?
B) What mass of barium sulfate will be produced?
Answer A): Limiting Reactant, Answer B): number unit of formula
#3
Answer A):
*For chemical formulas: enter subscripts as normal number, and no spaces.
◄ Previous
80
F3
JAN
24
$
4
Q
F4
%
5
Answer B):
9
F5
6
1
tv
c
F6
&
7
F7
* 00
8
DII
F8
- O
9
of
F9
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 1.99 g H2 is allowed to react with 10.3 g N2, producing 2.24 g NH3- Part A What is the theoretical yield in grams for this reaction under the given conditions? Express your answer to three significant figures and include the appropriate units. • View Available Hint(s) HÀ ? Value Units Submit Previous Answersarrow_forwardHelparrow_forwardHi! I need help with these limiting reactants questions from my Chem class pleasearrow_forward
- Looking for: Limiting Reagant Moles of each reactant leftover mass of each product madearrow_forwardPayalbenarrow_forwardThe illustration to the represents a mixture of iodine ( purple ) and fluorine ( green ) molecules. If the molecules in the above illustration react to form IF3 according to the equationI2 + 3 F2 2 IF3 , the limiting reagent is _______ The number of IF3 molecules formed is ________ , and the number of __________atoms/molecules in excess is __________ .arrow_forward
- When sodium chloride reacts with silver ni- trate, silver chloride precipitates. What mass of AgCl is produced from 61 g of AgNO3? Answer in units of g.arrow_forward14 of 19 Review I Constants I Periodic Table limiting reactant. Part B How many moles of PCI5 can be produced from 25.0 g of P4 (and excess Cl2)? Express your answer to three significant figures and include the appropriate units. View Available Hint(s) HA ? 202 mol Previous Answers Submit XIncorrect; Try Again; 5 attempts remaining Your answer is the number of moles of P4. Now use this value and the coefficient for PCI5 in the balanced chemical equation to find the number of moles of PCl,. P Pearson F12 II F8 F11 F10 F9 F7 F6 & delete 7 } P U enter II K return ? shift +IIarrow_forwardHow to calculate moles from a chemical reaction? e.g. the question below. Any info would help! 1. How many moles of H3PO4 are made when 1.45 moles of P4O10 react?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY