
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
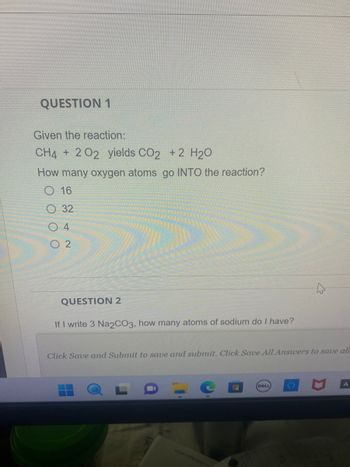
Transcribed Image Text:QUESTION 1
Given the reaction:
CH4 + 202 yields CO2 + 2 H₂O
How many oxygen atoms go INTO the reaction?
O 16
32
04
02
QUESTION 2
If I write 3 Na2CO3, how many atoms of sodium do I have?
Click Save and Submit to save and submit. Click Save All Answers to save all
H
DELL
A
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [References] ot A sample of calcium metal with a mass of 2.06 g was reacted with excess oxygen. The following equation represents the reaction that took place: ot 2Ca(s) + O2 (g) → 2CaO(s) The isolated product (CaO) weighed 2.25 g. What was the percentage yield of the reaction? ot ot Percentage yield otarrow_forwardQuestion 25 of 97 Submit DDT was an insecticide used to prevent mosquito borne diseases. Due to the harmful effects on birds and fish, it is now banned in the US. However, other countries still use it to control mosquitos. It can be prepared by the reaction shown below. Determine the amount of DDT that can be produced and the percent yield for this reaction. 2C6H,CI + C2HOCI, – C,4H9CI, + H20 2 3 NEXT If a company in South America started with 1175 g of chlorobenzene (C6H5CI, MW 112.55 g/mol) and 538.5 g of chloral (C2HOCI3, MW 147.39 g/mol), set up the table below that represents 100% yield with the given reaction conditions. 2C6H,CI C2HOCI, C1.H,Cls H2O Before (mol) Change (mol) After (mol) O RESET 3.132 -3.132 3.654 -3.654 7.308 0. + -7.308 10.44 -10.44 MacBook Air F12 II F8 F10 F11 000 F9 20 F3 F7 F4 F5 F6 esc F2 F1 +.arrow_forward5. An organic compound containing only C, H, and possibly O was subjected to combustion analysis. A sample weighing 0.7585 g yielded 1.295 g CO₂ and 0.464 g H₂O. What is the empirical formula of the compound?arrow_forward
- How many grams of Ab O3 will be created from reacting 36.0 g of Al with a sufficient amount of O2? 4Al(s) + 302(g) → 2Al¿Os(s)arrow_forwardIf a student burns 0.57 grams of methane (CH4) according to the equation below, what mass of carbon dioxide will be released? CH4 + 2 O2 --> CO2 + 2 H2Oarrow_forwardWhen 12.0 g of carbon is burned in oxygen during an experiment, only 39.8 g of carbon dioxide was collected. What is the percentage yield? C + O2 -> CO2arrow_forward
- A student was able to detect a mass increase of 0.500 grams during the reaction between Mg and O2. Oxygen was present in excess and the starting mass of Mg was 1.00 g. What is the percent yield of the reaction? O 50.0% O 90.9% O 150.% O 66.7%arrow_forwardStoichiometry Below is the generic skeleton chemical equation for a combustion reaction. A,B + C2 -> AC2 + B,C 1. What is the balanced equation for the combustion reaction?arrow_forwardYou are given the balanced equation: C3H8 + 5 02 3 CO2 + 4 H20 If 0.3618 moles of C3Hg are allowed to react with 1.818 moles of O2, and this is the only reaction which occurs, what is the theoretical yield of water (in moles) that could be produced? O 1.456 moles O 1.447 moles O 2.180 moles. O 1.454 molesarrow_forward
- When 5.0 g of propane are reacted with O2, carbon dioxide and water are produced. How many grams of H2O can be produced by the complete reaction of those 5.0 g of propane with excess oxygen? 20 g 1.1 g 8.2 g 0.45 g 2.04 garrow_forwardA3) Balance the following equation. You must enter a number in every blank. Fe(s) + O2lg) → Fe3O4(s)arrow_forwardFor the following question(s), consider the following balanced equation. Mg3N2 + 6H2O → 3Mg(OH)2 + 2NH How many grams of H2O are needed to produce 150 g of Mg(OH)2?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY