
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
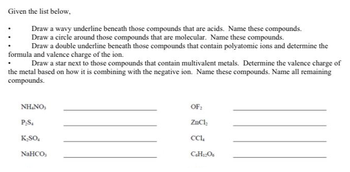
Transcribed Image Text:Given the list below,
Draw a wavy underline beneath those compounds that are acids. Name these compounds.
Draw a circle around those compounds that are molecular. Name these compounds.
Draw a double underline beneath those compounds that contain polyatomic ions and determine the
formula and valence charge of the ion.
Draw a star next to those compounds that contain multivalent metals. Determine the valence charge of
the metal based on how it is combining with the negative ion. Name these compounds. Name all remaining
compounds.
NH.NO,
P₂S.
K₂SO4
NaHCO,
OF:
ZnCl₂
CC1₁
C6H12O6
Expert Solution
arrow_forward
Step 1
Molecular compounds are the ones which are made up of only molecules.
Multivalent metals can exhibit more than one valency.
Polyatomic ions have more than one atom and can be either positively or negatively charge.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the name each binary covalent compound ?. Fill in the blanksarrow_forwardDo not give handwriting solution.arrow_forwardI’m trying to help my son with high school ninth grade chemistry. Could you please help us with this chart so we can study properly for his test. We do not understand cation and anion. arrow_forward
- For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will, then enter the chemical formula of the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 barium argon carbon element #2 compound formed? chemical formula O ionic O molecular O neither O ionic sulfur helium iodine O ο οίο ο ο molecular O neither O ionic O molecular Oneither 0 0 X Sarrow_forwardIndicate whether the following compounds are molecular or ionic and provide the systematic name. When naming the compound, do not include trivial names. If possible, include two systematic names (classic and Stock system names). If only one name is possible (not including trivial names), write it in the Stock section and write "not applicable" in the Classic section. Both name blanks ask for 2 words, the indication of a cation charge should be written without spaces right after the name of the cation. 1. CuF 2. CdI2 3. HI(aq) 4. NO 5. NF3arrow_forwardIf provided the name, then you must write the formula. If provided the formula, then you must write the correct name. Indicate IUPAC names only. Use one of the following descriptions: IONIC, COVALENT, ACID or HYDRATE. Compound Name or Formula Description 7. magnesium hydride 8. N2O 9. HBr (aq)arrow_forward
- Please provide the chemical formula that would form between the provided ions. Do not worry about using super- or subscript for your answer. Just insert any numbers that are necessary as normal-sized font. Combining Cu+ and O2- would form the chemical compound:arrow_forwardIonic Compounds, Covalent Compounds, and Chemical Equations a. Define the terms "ianic compound" and "covalent compound". Use all of the following terms correctly in at least one of the two definitions cation, anion metal, nonmetol, electrons are shared, and electrons are transferred. Write your answers in complete sentences. b. Complete the following chart using the information given. Correctly write the name or formula of each compound and identify it as lo covalent Formula AlO₂ K₂5 PC1₂ OF Name Magnesium iodide Tetrasulfur tetranitide Iron (III) sulfide Ammonium nitrate Tonico Covalent? CUCO Dinitrogen tetroxide c. Write o balanced chemical equation for the reaction that takes place between magnesium iodide and iron (III) sulfide ond identify the reaction typearrow_forwardSubmitting 2nd time as I was asked to resubmit to obtain help with the rest. Thank you.arrow_forward
- Indicate whether the following compounds are molecular or ionic and provide the systematic name. When naming the compound, do not include trivial names. If possible, include two systematic names (classic and Stock system names). If only one name is possible (not including trivial names), write it in the Stock section and write "not applicable" in the Classic section. Both name blanks ask for 2 words, the indication of a cation charge should be written without spaces right after the name of the cation. 1. CuF 2. CdI2 3. HI(aq) 4. NO 5. NF3arrow_forwardCHEM& 121 Highline Beaulac 12) Write the chemical formulas of the following compounds. Iron (III) oxide Ammonium sulfate Potassium cyanide Sodium carbonate Calcium nitrate Copper (II) hydroxide Aluminum Sulfite Strontium phosphate 13) Write the correct chemical names for the following compounds. Circle whether they are ionic or covalent. Ag20 Ionic Covalent NazSO4 Ionic Covalent NH4OH Ionic Covalent Au2(CO3)3 Ionic Covalent K3PO3 Ionic Covalent P2O5 Ionic Covalent ZnBr2 Ionic Covalent Pb(CN)2 Ionic Covalent N20 Ionic Covalentarrow_forwardPlease help which one is right?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY