
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
make sure to type this out, please don’t write it on paper!
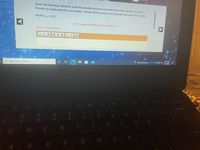
Transcribed Image Text:Given the following reactants, write the possible products and determine if the reaction will occur.
Provide an explanation for your answer. Include which products are insoluble and which are soluble.
Pb(NO3)2 + K2S
Use the paperclip button below to attach files,
Student can enter max 3500 characters
B I
U
O Type here to search
1205 AM
Rain off and on aD *
ロ
3/12/2022
End
Delee
Home
Backspace
%23
7
5
3
Y
WE R
Enter
A
S D F G H J K L
414
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- U guys gotta help me on this !!arrow_forwardUsing family suffixes to name organic compounds Name these organic compounds: structure name H. H–C– OH H. CH, — СН,— СH, –C – H || Н— С — ОН Check Explanation O 2021 McGraw Hill LLC. AlI Rights Reserved. Tern ||arrow_forwarda_____ is a group of atoms bonded in a specific pattern and is the reactive portion of an organic molecule .arrow_forward
- = Organic Molecules Assignment Draw the structure of iodobenzene. Check Click and drag to start drawing a structure. Terms of Use | Privacy Center Accessibility C™ X A ċ Save For Later tu Submit Assignmentarrow_forwardHow can we increase the stability of droplet in technical DSMEarrow_forward_Na2SO4 + _AlCl3 => _Al2(SO4)3 + _NaClarrow_forward
- 7. You can see an MSDS below. Please answer the following questions related to the MSDS. a) What is the name of this chemical? b) What should you do if someone drinks the chemical? c) Would this chemical catch on fire if it was exposed to flames? d) If this chemical gets in your eye what should you do? e) What color is this chemical? f) What should you do if someone spills a small amount of the chemical?arrow_forwardI don't understand as I can't read the writing. Can you type this please?arrow_forwardhelp mearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY