Question
Given the following hybrid orbitals, which are orthogonal but not normalized: image
It is requested to obtain their expressions in normalized form.
Date: The s and px atomic orbitals are orthonormal, that is, they meet the orthogonality and normalization conditions.
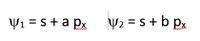
Transcribed Image Text:Y1 = s+ a px
Y2 = s + b px
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- The value of (p,H) the square bracket represents the poison brackets, p is the density of states and H is the hamiltonian isarrow_forwardQuestion1: Normal Zeeman, which is formed when an atom is placed in an external magneticfield environment, Abnormal Zeeman, Electron spin resonance and Paschen Back eventsWrite down the Hamiltonians in tabular form to express their differences.arrow_forwardA conduction electron is confined to a metal wire of length (1.46x10^1) cm. By treating the conduction electron as a particle confined to a one-dimensional box of the same length, find the energy spacing between the ground state and the first excited state. Give your answer in eV. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answerarrow_forward
- Explain how Cooper pairs can exist in superconducting materials, even though electrons repel each other.arrow_forwardTo enhance ionized impurity scattering limited mobility and phonon scattering limited mobility, how you will change doping concentration (increase or decrease) and lattice temperature (increase or decrease)? Is the peak of the mobility value determined by ionized impurity scattering limited mobility value or phonon scattering limited mobility value at a particular temperature?arrow_forwardWrite down the ground state electronic structure for Rb by inserting the correct integer numbers in the boxes below. Leave a box empty, or insert a zero if you want to indicate no electrons occupy that specific orbital. You can look at the Periodic Table (Section 5.4 of Unit 21) to establish the number of electrons in Rb. 1s 2s 2p 3s 3p 4s 3d 4p 5s 4d Select from the list below one element that you expect would have similar chemical properties to Rb. ○ Li Mg OCI B S Asarrow_forward
- Explain the reason that metal atomic chains have quantized G, different from bulk metals by comparing with the Schematic illustration of a diffusive and ballistic conductor.arrow_forwardpls send me answer of this question in written form with expalantion and i will give you like sure Draw the projections for spin angular momentum. Calculate the angles between the z-axis and the spin angular momentum S of the electron in the up and down spin states.arrow_forwardWrite down the confi gurations for the ground states of calcium and aluminum. What are the LS coupling quantum numbers for the outside subshell electrons? Write the spectroscopic symbol for each atomarrow_forward
- Please, I want to answer the question correctly and clearly for this question, the name of the course: Laser and its applicationsarrow_forwardConsider a hydrogen-like atom such as He+ or Li++ that has a single electron outside a nucleus of charge Ze. (a) Rewrite the Schrödinger equation with the new Coulomb potential. (b) What change does this new potential have on the separation of variables? (c) Will the radial wave functions be affected? Explain. (d) Will the spherical harmonics be affected? Explain.arrow_forwardDescribe the tunneling of superconducting cooper pairsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios