
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
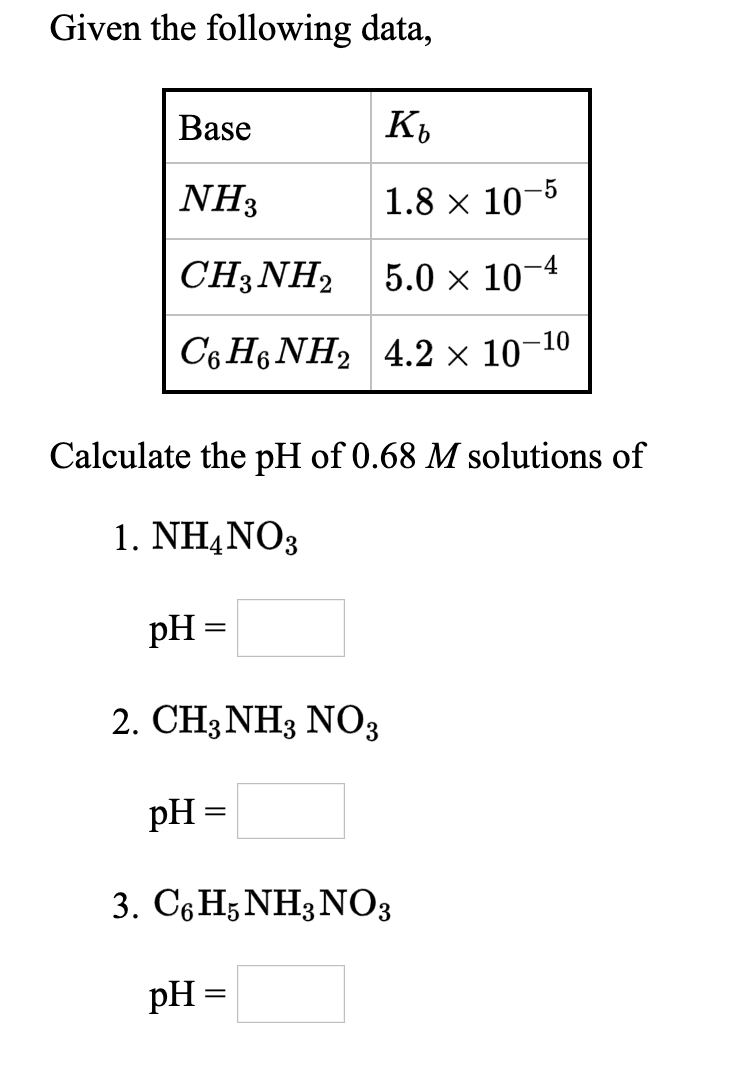
Transcribed Image Text:Given the following data,
Base
K,
NH3
1.8 x 10-5
CH3NH2
5.0 x 10
C6 H6 NH2 4.2 × 10-10
Calculate the pH of 0.68 M solutions of
1. NH4NO3
pH =
2. CH3NH3 NO3
pH =
3. СеHsNH3NОз
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Similar questions
- Calculate the pH of each of the following solutions. Ка (НС, Н3О2) %3D1.8 х 10-5 Kа(НCN) 3D 6.2х 10-10 Къ (NH3) — 1.8х 10-5 а. 0.2 М КС2Н3О2 pH = b. 0.51 M NаCN pH = с. 0.4 М NH4 CІ pH =arrow_forwardEach value represents a different aqueous solution at 25 °C. Classify each solution as acidic, basic, or neutral. Incorrect Acidic pH = 4.57 pOH = 4.97 pOH = 9.52 [H+] = = 9.8 x 10-5 Basic pH = 9.45 [H+] = 5.5 x 10-¹¹ [OH-] = 4.9 × 10-¹1 [OH-] = 5.4 x 10-² Answer Bank Neutral [H+] = 1.0 x 10-7 pOH = 7.00arrow_forwardDetermine [OH–], pOH and pH of a 0.63 M aqueous solution of LiC3H5O3 (K b = 7.1×10-11).arrow_forward
- Identify whether each species functions as a Brønsted-Lowry acid or a Brønsted-Lowry base in this net ionic equation. CH;C00 HCN (aq) CHĄCOOH CN Brønsted-Lowry | Brønsted-Lowry Brønsted-Lowry | Bronsted-Lowry { In this reaction: The formula for the conjugate )of CH3COO¯ is The formula for the conjugate| of HCN isarrow_forward[H3O+1] = 10-PH [OH-1] = 10-pOH [H3O+1][OH1] = 10-14 pH - log [H3O+1] = %3D pOH = - log [OH-] pH + pOH = 14 %3D %3D What will be the pH if 0.00150 moles of HCl is added to 1.00L of water ? The reaction gives the conversion factor HCI + H2O H3O+1 + CI1 start: 0.00150 equil: O A. 1.5000 В. 2.5223 C. 2.824 D. 3.125 Е. 6.502arrow_forwardN2H4 is a weak base. approximately how many moles of N2H4 are required for each mole of N2H5+ to create a solution with a pH equal to 8.5?arrow_forward
- Consider 0.100 M aqueous solutions of the following chemicals. Rank them in increasing order of pH (lowest pH to highest pH). HCIO₂ Rationale Ranking: NaF CH3CH₂NH3CI KCH3COO NH3arrow_forward1 M NaOH has the same pH as 1 M NH4OH. O True O Falsearrow_forwardThe ?a of propanoic acid (C2H5COOH) is 1.34×10−5. Calculate the pH of the solution and the concentrations of C2H5COOHand C2H5COO− in a 0.515 M propanoic acid solution at equilibrium.arrow_forward
- Determine the [OH-], pH, and pOH of a solution with a [H+] of 4.7 × 10-¹² M at 25 °C. [OH-] = pH: = POH Determine the [H+], pH, and pOH of a solution with an [OH-] of 6.7 x 10-7 M at 25 °C. [H+] = pH : = = POH = M Marrow_forwardNEED HELParrow_forwardWhich of these acids has the lowest pH at equilibrium? Acid Ka HIO3 1.7 x10-1 HCIO2 1.1 x10-2 HCOOH 1.8 x10-4 CH3COOH 1.8 x10-5arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY