
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
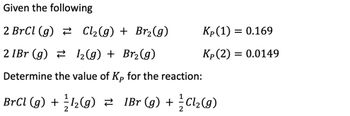
Transcribed Image Text:Given the following
2 BrCl (g)
Cl₂(g) + Br₂(g)
2 1Br (g)
1₂(g) + Br₂(g)
Determine the value of Kp for the reaction:
Kp (1) = 0.169
Kp (2) = 0.0149
BrCl (g) + 1₂(g) ≥ 1Br (g) + ²Cl₂(g)
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction, H2(g) + I2(g) ⇌ 2 HI(g), Kp = 44 at 25.0 o C. Calculate the value of Kcarrow_forwardKp for the following reaction is 0.16 at 25 0C. 2NOBr(g)=2NO (g)+ Br2 (l) the enthalpy change for the reaction at standard conditions is +16.1 kJ. State which way the equilibrium will shift (left, right or no change) when each of the following changes is made. Breifly explain credit for result. (a) adding more Br2 (l) (b) removing some NOBr (g) (c) Decreasing the temperature (d) increasing the container volume (e) increasing the total pressure by adding an inert gas.arrow_forwardThe equilibrium constant, Kc, for the following reaction is 1.29×10-2 at 600 K.Calculate Kp for this reaction at this temperature.COCl2(g) CO(g) + Cl2(g)arrow_forward
- Provide an expression for KC and K . This is not a numerical answer.Express KP with respect to KC . This is not a numerical answer.arrow_forwardFor the equilibrium N2O4(g) <----> 2 NO2(g), at 298 K, Kp = 0.15. For this reaction system, it is found that the partial pressure of N2O4 is 3.7 × 10^–2 atm at equilibrium. What is the partial pressure of NO2 at equilibrium?arrow_forwardHydrogen chloride and oxygen react to form water and chlorine, like this: 4HCl(g) + O2(g) →>> 2H2O(g)+2Cl2(g) Suppose a mixture of HCl, O2, H2O and Cl2 has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation change in composition shift in equilibrium to the right The pressure of HCl will ? Some Cl₂ is added. to the left The pressure of O₂ will ? ○ (none) to the right The pressure of O2 will ? Some HCl is removed. to the left The pressure of H2O will ? O (none)arrow_forward
- Hydrogen chloride and oxygen react to form water and chlorine, like this: 4HC1(g) + O₂(g) → 2H₂O(g) + 2Cl₂(g) Suppose a mixture of HC1, O2, H₂O and Cl₂ has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation Some HCI is removed. Some Cl₂ is added. change in composition The pressure of O₂ will The pressure of H₂O will The pressure of HCI will The pressure of O₂ will ? ? ? ? ↑ î î shift in equilibrium X O OOO to the right to the left (none) to the right to the left (none) Śarrow_forwardAt 487 K, this reaction has a Ke value of 0.0463. 2 X(g) + 3 Y(g) = 2Z(g) Calculate Kp at 487 K. Note that the pressure is in units of atmosphere (atm). Kp =arrow_forwardThe equilibrium constant, Kċ, for the following reaction is 1.29×10-² at 600 K. Calculate Kp for this reaction at this temperature. COCI₂(g) ⇒CO(g) + Cl₂(9) Кр || =arrow_forward
- Acetylene and oxygen react to form carbon dioxide and water, like this: 2C,H,(g)+50,(g) → 4CO,(g)+2H,0(g) Suppose a mixture of C,H2, O2, CO, and H,O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. olo perturbation change in composition shift in equilibrium Ar to the right The pressure of C,H, will v ? to the left Some H,0 is removed. go up. The pressure of O, will (none) go down. not change. to the right The pressure of O, will ? to the left Some C,H, is added. The pressure of CO, will O (none)arrow_forward10arrow_forwardThe following equilibrium constants were determined at 1123 K: Kp=7.79 × 10 Kp"=1.67 × 10² 2CO(g)C(s) + CO₂(g) COC1₂(g) CO(g) + Cl₂ (g) Part 1 of 2 Calculate the equilibrium constant at 1123 K for the reaction: 2COC1₂ (g) C(s) + CO₂(g) + 2Cl₂ (g) Round your answer to 3 significant digits. Part 2 of 2 n x10 = Kp² X Write the equilibrium constant expression: X Olo 15arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY