
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

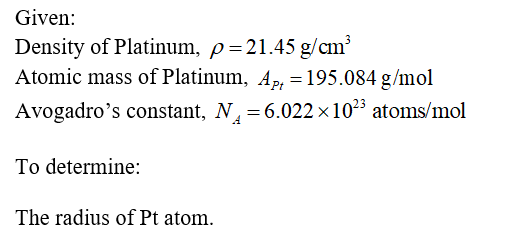
Transcribed Image Text:Given that Pt has an FCC crystal structure, density (p) =21.45 g/cm³, atomic mass (Ap) = 195.084
g/mol, Avogadro constant (NA) ~ 6.022 × 1023 atoms/mol. Calculate the radius of the Pt atom.
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Show that Ni and Cu are totally soluble in one another using Hume-Rothery rules. Atomic radii, electronegativities and crystal structures of Ni and Cu are given below. Ni Cu Crystal Structure FCC FCC Electronegativities 1.9 1.8 r (nm) 0.1246 0.1278arrow_forwardIn an ideal hexagonal closed packed system, if the atomic radius of the atom was 0.112 nm, write down the three digit value of the lattice parameter c in nm unitarrow_forwardCalculate the density in g / cm3 of a metal with a structure fcc molecular mass of 100g / mol and an atomic radius of 0.145nmarrow_forward
- 14. In an ideal hexagonal closed packed system, if the atomic radius of the atom was 0.112 nm, write down the three digit value of the lattice parameter cin nm unit Enter your answerarrow_forwardConsider a unit cell with lattice dimensions a, b, and c in the x-, y and z directions. Which of the following sets of Miller indices can represent a crystallographic plane with intercepts of b and (c/4) along the y- and z-axes respectively? (more than one answer is possible for this question. marks will only be awarded for the question if all the correct options are selected (i.e. 'all or nothing' marking scheme). a. b. C. d. (214) (421) (114) e. (412)arrow_forwardConsider two hypothetical metallic crystal structures A and B. A has a simple cubic and B a fcc lattice structure. Both have the same unit cell volume. The Atomic Packing Factor for A is 0.520 and that for B is 0.740. What is the ratio of the fcc to simple cubic atomic radii?arrow_forward
- Estimate the density of platinum and lead from their lattice parameters at room temperature. Both are FCC. Compare the theoretical density with experimental values. Which is closer? Why? For platinum A=192.09, Avogadro number =6.02x1043, a=3.9239 Angstrom. Experimental density of Pt = 21.47. For lead A=207.2, and a=4.9502Angstrom.arrow_forwardplease answer quicklyarrow_forward5. Calculate Phkl for the (100), (110), and (111) planes of a monoatomic fcc crystal.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY