
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Give the oxidation state of each metal species.
Fe−→−−−−−heatexcess COFe(CO)5−→−I2heatFe(CO)4I2
Expert Solution
arrow_forward
Step 1
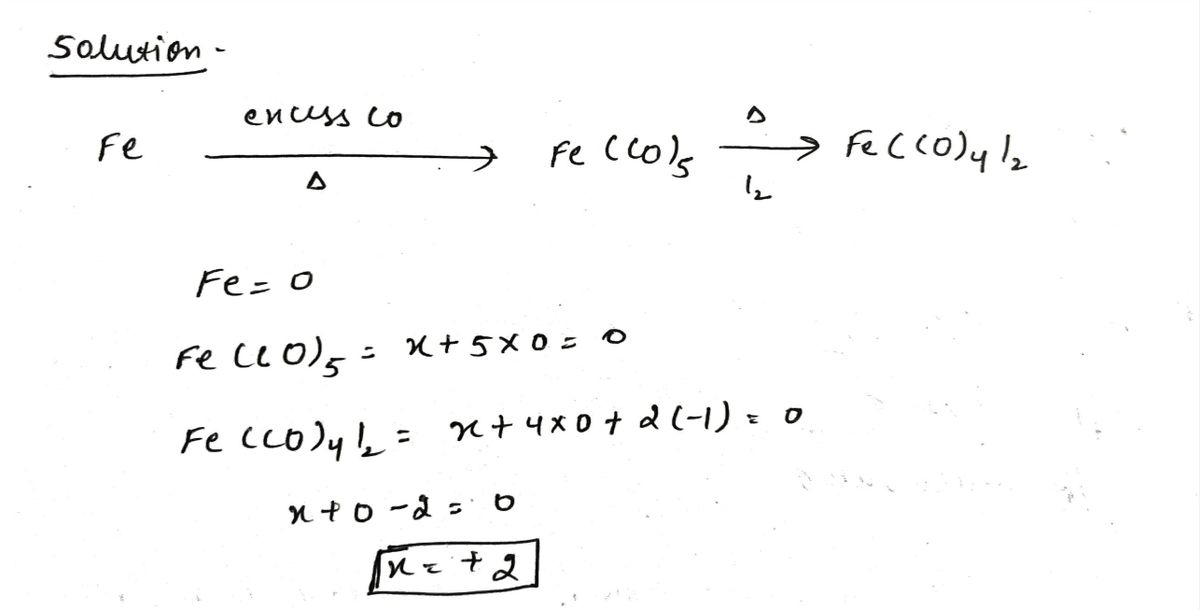
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- what type reaction is 2Fe+3H2SO4=Fe2(SO4)3+2H2arrow_forward14.4 g of magnesium nitrate is dissolved in 1.20 L of water. 25 mL of this solution is taken and diluted to 175 mL with water. What is the final concentration of the nitrate ion?arrow_forwardDescribe in words how you do the following preparation. Then give molecular equation for the preparation. MgCl2(s) from Mg(OH)2(s)arrow_forward
- 1. If you use 6.95 g of the CuSO 5 H₂O starting material, what mass in grams of the [Cu(NH3)4]SO4 H₂O product would you expect to isolate at the end of the reaction? Include 3 significant figures in your answer. This mass represents the theoretical yield of the product.arrow_forwardWrite the balanced NET ionic equation for the reaction when (NH4)2CO3 and CaCl2 are mixed in aqueous solution. If no reaction occurs, simply write only NR.arrow_forward7 The oxidation state of chlorine in ClO4- is A) 0 B) +5 C) -5 D) +7arrow_forward
- 10. Write the completed and balanced equation with the correct physical state for each species in molecular, ionic and net ionic form. CS3PO4 (aq) + Ge(C2H3O2)4 (aq) → lonic: Net lonic:arrow_forwardA yellow solid is either lead(II) chromate or sodium chromate. What would you do to tell which compound the solid is?arrow_forwardConsider the series of reactions to synthesize the alum (KAl(SO4 )2 · xH2O(s)) Assuming an excess of the other reagents, from one mole of aluminum Al (s), how many moles of alum will be produced?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY