
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
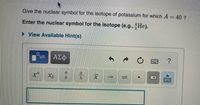
Transcribed Image Text:Give the nuclear symbol for the isotope of potassium for which A = 40 ?
Enter the nuclear symbol for the isotope (e.g., He).
• View Available Hint(s)
ΑΣφ
?
a
Xb
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Does any element with ZS 92 match the description? If you checked yes, give the symbol of an element with Z S 92 description that matches. An element in Period 4 and Group 3A. O Yes O No A metalloid in Group 5A. O Yes O No A halogen with a higher atomic number than silicon. O Yes O No A main-group element in Period 2. O Yes O No Oarrow_forwardNonearrow_forwardPlease only answer part b. Thank you! (b) The negative muon (μ−) behaves like a heavy electron, with the same charge as the electron but with a mass 207 times as large as the electron mass. As a moving μ− comes to rest in matter, it tends to knock electrons out of atoms and settle down onto a nucleus to form a "one-muon" atom. For a system consisting of a nucleus of platinum (Pt195 with 78 protons and 117 neutrons) and just one negative muon, predict the energy in eV of a photon emitted in a transition from the first excited state to the ground state. The high-energy photons emitted by transitions between energy levels in such "muonic atoms" are easily observed in experiments with muons.arrow_forward
- Recent Modules 7 of 106 Part A When a U nucleus is bombarded by neutrons (n) it undergoes a fission reaction, resulting in the formation of two new nuclei and neutrons. The 92 following equation is an example of one such fission process: Un Ba Kr + Enter the isotope symbol for the barium (Ba) nucleus in this reaction. Express your answer as an isotope. > View Available Hint(s) ΑΣφ Submitarrow_forwardQuestions 3 and 4 And the check the othersarrow_forwardI need help with these true or false statementsarrow_forward
- An atom of rhodium (RhRh) has a diameter of about 2.7×10−8cm2.7×10−8cm.arrow_forward$ 4 Q F4 R Part A F What is the mass of 5.08x1021 platinum atoms? View Available Hint(s) Provide Feedback m= Submit [5] ΑΣΦ % 5 g F5 T G 6 MacBook Air J F6 C Y & 7 H F7 ? U g * 00 8 J DII FB ( 9 Karrow_forwardAnswer correctly I’m cursivearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY