
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
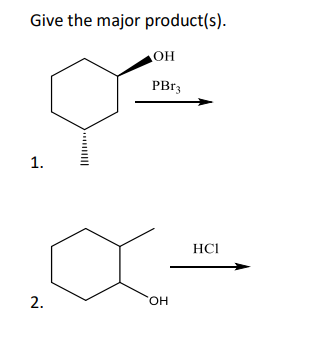
Transcribed Image Text:Give the major product(s).
OH
PBr3
1.
2.
OH
HCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps with 2 images

Knowledge Booster
Similar questions
- Nitrate2arrow_forwardIn the following reaction, what is the overall reaction product(s)? CH 4+2O 2→CO 2+2H 2O a. CO2+2H2O b. CO2 c. CH4+2O2arrow_forward26. 2Kl(aq) + H2O2(l) ↔ 2KOH(aq) + I2(g) + O2(g)Write the reaction above in total ionic form, and identify the spectator ion(s). The dissolved substances (designed by aq) are the only ones that dissociate completely into ions. a. I - b. K + c. OH - d. more than one choice is correctarrow_forward
- Please don't provide handwriting solutionarrow_forwardAntacids neutralize the hydrochloric acid in your stomach. What is the correct neutralization reaction of stomach acid with Milk of Magnesia (Mg(OH)2)(Mg(OH)X2) ? Select one: HCl+Mg(OH)2⟶H2O+MgCl2HCl+Mg(OH)X2⟶HX2O+MgClX2 2HCl+Mg(OH)2⟶2H2+O2+MgCl22HCl+Mg(OH)X2⟶2HX2+OX2+MgClX2 HCl+Mg(OH)2⟶HMg+ClOHHCl+Mg(OH)X2⟶HMg+ClOH 2HCl+Mg(OH)2⟶2H2O+MgCl22HCl+Mg(OH)X2⟶2HX2O+MgClX2 HCl+Mg(OH)2⟶HMg+2Cl(OH)2arrow_forwardIs this correct ?arrow_forward
- For the following reaction, write the balanced molecular equation, the complete ionic equation and the net ionic equation. Making sure that the equations are balanced. Must give at least three equations.arrow_forwardWhat is the balanced equation for salicylic acid + acetic anhydride --> acetylsalicylic acid (aspirin) + acetic acid? When added, why is concentrated sulfuric acid a catalyst?arrow_forwardConsider the reaction below: 3 Na2O(aq) + 2 Al(NO3)3(aq) ----> _ Compound A + _ Compound B (OR "3" N a2O(a q) + "2" A l(N O3)3(a q) right arrow Compound A + Compound B) One of the the single ions that would be used to form compound A or compound B would be ____.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY