
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working
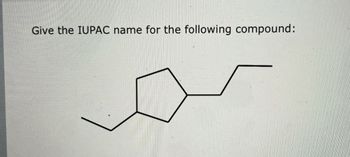
Transcribed Image Text:Give the IUPAC name for the following compound:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardREPORT FORM Experiment #9 – Analysis for Ions Name Section Date Unknown Sample # ΧYΖ PART A: CATION TESTS OPERATION OBSERVATION CONCLUSION Section Flame test for Na", K* and Ca“ ions NaCl Bright yellow KCI Lavender |CaCl, Not very evident Flame test for unknown Bright yellow Section 2: Test for Ca ions |(NH,),C,O̟with NaCl |(NH,),C,O, with KCI NH,),C,O, with CaCl, |(NH,),C,O,with FeCl, Clear solution Clear solution White precipitate Clear solution |(NH),C,0̟with unknown Clear solution Section 2: Test for Fe" ions KSCN with NaCl Clear solution KSCN with KCI Clear solution KSCN with CaCl, Clear solution KSCN with FeCl, Blood red precipitate KSCN with unknown Clear solution The unknown cation isarrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Hello, I hope you are doing well on this fine day. For the following quetion please read carefully the question and instruction. PLEASE ANSWER QUESTION IN 20 MINTUES NOT MORE PLEASE AND THANK YOU. If you do answer the question correctly and post it in the next 20 minutes, NO NEED TO SHOW ALL THE WORK, I JUST WOULD LIKE THE CORRECT ANSWER AS SOON AS POSSIBLE. I will write a wonderful and generous feedback/review/rating about you.arrow_forward= teaching and lea x /ilm/takeAssignment/takeCovalentActivity.do?locator-assignment-take juju_ arch CuO chemical name - Google Se xb Answered: A 30.7 ml sample of = x + Free Online Survey... Submit Answer Home - Academia... Create Your Rubric... The Utility Experts .... [Review Topics] [References] Use the References to access important values if needed for this question. A 24.3 mL sample of a 0.318 M aqueous nitrous acid solution is titrated with a 0.405 M aqueous barium hydroxide solution. What is the pH at the start of the titration, before any barium hydroxide has been added? pH = Retry Entire Group 8 more group attempts remaining hp 16 Quando a rede soci... # 0 » NEW * 4x Carrow_forwardQUESTION 21 The equilbrium constant, Kc, for the following reaction is 115 at some temperature. H2 (g) + F2 (g) ⇌ 2HF (g) If 2 M H2 and 2.25 M F2 are sealed in a flask, what will be the equilbrium concentration of HF? Report your answer to 3 significant figures.arrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardchemistry lab please write the answer in the question paper the one I uploaded it thank youarrow_forward
- Draw the products of the reactions. products Select Draw Rings More Erase H + H,0 H+ products Select DrawRings More Erase H about us careers privacy policy terms of use contact us helparrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardsession.masteringchemistry.com/myct/itemView?assignment ProblemID=190556628&offset=next Discussion Question 1: Chapter 6 Homework blem 6.20- Enhanced - with Feedback Enter the symbols for the ions and the correct formula for the onic compound formed by each of the following. esc Q A N F1 2 W S F2 X #3 20 E D F3 с S4 $ OOO 000 R Part E F4 F Enter the symbols for the ions of sodium and phosphorus. Enter the lons formed by these elements and separate your answers with a comma (e.g., Sr²+, As³-). cation, anion = Part F Complete previous part(s) Submit Part G cation, anion Submit Part H Complete previous part(s) 65° V Enter the symbols for the ions of calcium and sulfur. Enter the lons formed by these elements and separate your answers with a comma % Request Answer T Request Answer G 6 ΑΣΦ B ΑΣΦ MacBook Air F6 Y H 18⁰ h & 7 N F7 U * CO 8 J BUT RAS poddany ▶II ? F8 1 ? E 9 K F9 O L (e.g., Sr²+, As³-). 0 Ga3+ and 02-E L F10 P F11arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY