
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
22) Give systematic names for the following binary compounds.
CsF
K2O
CuO
Expert Solution
arrow_forward
Step 1
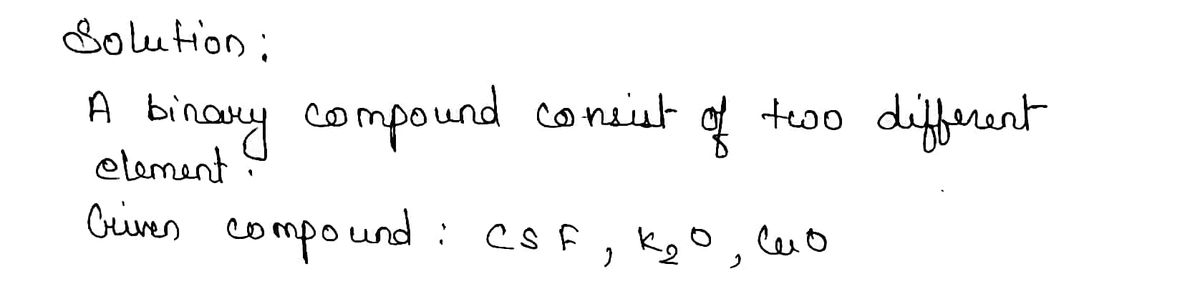
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 11 Provided the chemical names for the following compounds with transition metals, provide their molecular formulas Tin(IV) sulphide Iron(ll) phosphide Zinc(IIl) phosphatearrow_forward1) Pretend there is a new metal wagium with the symbol Wg, and it is a variable charge metal. It makes the compound WgN. What is the name of this compound? What type of compound is this? What is the charge on the metal? 2) Pretend there is a new metal Purrium with the symbol Pi, and it is a fixed charge metal. It makes the compound Pi(SO4)2. What is the name of this compound? What type of compound is this? What is the charge on the metal? How are the nomenclature rules different for these two compounds?arrow_forwardChoose the name / formula pair that does not correctly match. divanadium pentoxide /V₂05 magnesium hydroxide / Mg(OH)2 iron (II) sulfate / FeSO4 chlorine monoxide / CIOarrow_forward
- Predict the chemical formula for the ionic compound formed by the ions Bal+ and C₂H3O2¯. tharrow_forwardWhat is the chemical formula of the following compound? What would be possible identities of the anion and cation?arrow_forwardChoose the name / formula pair that does not correctly match. ammonium sulfide / (NH4)2S magnesium hydroxide / Mg(OH)2 aluminum phosphate / AIPO4 calcium acetate / CaCH3COO zinc carbonate / ZnCO3arrow_forward
- Predict the chemical formula for the ionic compound formed by Ca²⁺ and ClO₃⁻arrow_forwardFind the ionic compound among these. SO3 O C H4 CaH2 О CаН2 O C2H6arrow_forwardwrite the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Ca2+, Al3+, Br-, O2-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY