
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give detailed Solution with explanation..give correct answer please
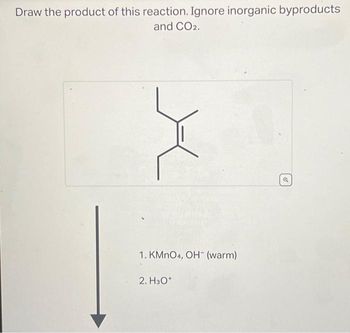
Transcribed Image Text:Draw the product of this reaction. Ignore inorganic byproducts
and CO2.
X
1. KMnO4, OH-(warm)
2. H3O+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Example Problem: Consider The Titration of 100.0 mL of 0.200 M CH3NH2 by 0.100 M HCl. Calculate The pH After 65.0 mL of HCl Added. (Kb for CH3NH2 = 4.4 x 10-4): 10.96 - correct answer. 9.75. 7.00. 3.68. 2.54.arrow_forwardif an acidic functional group is added with a basic functional group, the resulting structure will always be a neutrally-charged molecule provide logical reasoning that this is truearrow_forwardi need help to rank each 5 compunds by relative basicity use 1 for the strongest base all the way 5 which is the weakst base please I really need to get this queshtion right 1. 2. 2. 4. 5.arrow_forward
- Tab Window Help Learning Xx AT HOMEWORK Module 9 X M hydrochloric acid Submit Answer MindTap Cengage Learning mentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786068&snapshotld-33225... References Use the References to access important values if needed for this question. : Concentration Acid/Base: This is group attempt 1 of 10 tv An aqueous solution of hydrochloric acid is standardized by titration with a 0.188 M solution of barium hydroxide. X If 23.6 mL of base are required to neutralize 16.7 mL of the acid, what is the molarity of the hydrochloric acid solution? G What volume (in mL) of a 0.125 ☆ Autosaved at 10:58 PM Q A ·arrow_forward[References] Write the formula for the conjugate base of NH,".arrow_forwardGive correct detailed Solution with explanation needed with structure ..don't give Handwritten answer..don't use Ai for answering thisarrow_forward
- Please don't provide handwritten solution ......arrow_forwardDraw a picture of a proton transfer reaction including curved arrowsarrow_forwardThe concentration H*| of a substance is 10 10. Calculate the pH value and classify it as an acid or a base. ... The pH value of the given substance is (Simplify your answer.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY