
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
5. From the possible compounds listed in the Excel file, determine the Unknown Hydrocarbon.
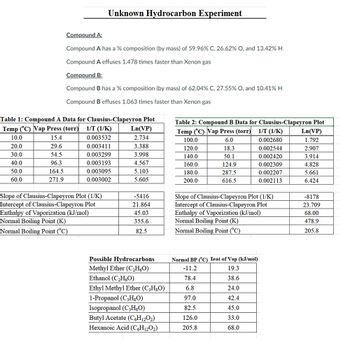
Transcribed Image Text:### Unknown Hydrocarbon Experiment
#### Compound A:
- **Composition (% by mass):** 59.96% C, 26.62% O, 13.42% H
- **Effusion Rate:** 1.478 times faster than Xenon gas
#### Compound B:
- **Composition (% by mass):** 62.04% C, 27.55% O, 10.41% H
- **Effusion Rate:** 1.063 times faster than Xenon gas
---
#### Table 1: Compound A Data for Clausius-Clapeyron Plot
| Temp (°C) | Vap Press (torr) | 1/T (1/K) | Ln(VP) |
|-----------|------------------|-----------|--------|
| 10.0 | 15.4 | 0.003532 | 2.734 |
| 20.0 | 29.6 | 0.003411 | 3.388 |
| 30.0 | 54.5 | 0.003299 | 3.998 |
| 40.0 | 96.3 | 0.003193 | 4.567 |
| 50.0 | 164.5 | 0.003095 | 5.103 |
| 60.0 | 271.9 | 0.003002 | 5.605 |
**Summary of Clausius-Clapeyron Plot for Compound A:**
- **Slope:** -5416 (1/K)
- **Intercept:** 21.864
- **Enthalpy of Vaporization (kJ/mol):** 45.03
- **Normal Boiling Point (K):** 355.6
- **Normal Boiling Point (°C):** 82.5
---
#### Table 2: Compound B Data for Clausius-Clapeyron Plot
| Temp (°C) | Vap Press (torr) | 1/T (1/K) | Ln(VP) |
|-----------|------------------|-----------|--------|
| 100.0 | 6.0 | 0.002680 | 1.792 |
| 120.0 | 18.3 |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 17 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the name of this molecule?arrow_forwardLabel the functional groups in the molecule. Answer Bank hydroxy group Br alkoxy group HO halo group amino group NH2 H2Narrow_forwardA Moving to another question will save this response. estion 18 Which of the following is NOT a correct name for the alkane shown with it? (Drawing the structures wilI help!) O C7H16, heptane О С2Н6, ethane O C10H22, decane CH4, methane O C3H12. propane Moving to another question will save this response. MacBoarrow_forward
- Write the name of each compound underneath the corresponding stucture.arrow_forwardTo name carboxylic acids use the name of the pare “_ Oi C 0ic " at the end. Example: Name the following: H. .C. C C. HO H. H. 188 エ エー○ エIarrow_forward6. Name 5 functional groups in the given molecule. obcomm HO NH₂arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY