
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Is my stereochemistry correct? I don’t know which carbon the the acid attacks so I don’t know if I have the right stereochemistry. It has to look like the top one.
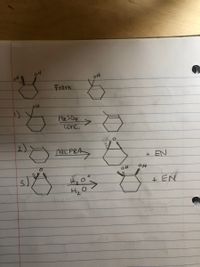
Transcribed Image Text:04
From
Hz SO4
conc.
2)
mcPBA
EPEAS
+EN
+ EN
4.
3)
->
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Only typed solutionarrow_forward13arrow_forwardUse the information in the pK₂ table below to determine which side of the equilibrium is favored for each of the reactions in the second table. acid pK₂ acid pk₁ H₂ NH₂ X OH NH3 NH CH,SH₂ CH₂- 36 36 15.9 10.8 5.3 N + NH₂ OH + CH₂OH + CH,NH, CH₂OH CH₂-S -OH O OH Š CH₂SH₂ CH3NH 36 ΝΗ 19 15.7 9 Equilibrium Equation -2 -7.2 NH CH₂0 + H CH₂- NH₂ -fo + CH, SH + CH,NH, + NH₂ Left Equal Favored O O O O O O Right Favored O O O O Oarrow_forward
- 2. What products would be obtained by treating A-D with NaOt-Bu in t-BuOH? Explain your reasoning, and if more than one product forms, indicate which product is major. Br A Br B C لي Br Darrow_forwardDraw the major organic product(s) of the following reaction. Br + NaCN DMF • You do not have to consider stereochemistry. • If no reaction occurs, draw the organic starting material. • When SN1 & E1 pathways compete, show both the substitution and the elimination products. Separate multiple products using the + sign from the drop-down menu. .Do not include counter-ions, e.g., Na+, I, in your answer. ? n ChemDoodlearrow_forwardWhy is compound la stonger base than compound I? H - H N. II O 1. Because compound II is more sterically hindered. O2. Because compound I is more stable than compound II. O 3. Because the lone pair on compound Iis part of a delocalized pi-system. O4. Because the lone pair on compound II is part of a delocalized pl-system.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY